Abstract
Objective
Cisplatin is a widely used chemotherapeutic agent in the treatment of cancers in clinic; but it often induces adverse effects on ovarian functions such as reduced fertility and premature menopause. Mesna could attenuate the cisplatin-induced ovarian damages; however, the underlying mechanism is still unknown. This study aimed to figure out the underlying mechanism of the protection of mesna for ovaries against cisplatin therapy in cancers.
Methods
We performed female adult Sprague-Dawley rats into normal saline control (NS), low-dose cisplatin (CL), high-dose cisplatin (CH), CL plus mesna (CL+M), and CH plus mesna (CH+M) groups and detected anti-Müllerian hormone (AMH)-positive follicle, oxidative stress status and anti-oxidative capability in ovaries.
Results
AMH-positive follicles were significantly decreased after cisplatin administration, which was significantly reversed when mesna was co-administered with cisplatin. The end product of lipid peroxidation, malondialdehyde (MDA), was significantly increased, but the anti-oxidative enzymatic activity of superoxide dismutase (SOD) and glutathione (GSH) were significantly decreased in cisplatin groups when compared with NS group. In contrast, after co-administration of cisplatin with mesna, MDA was significantly decreased whereas the activity of SOD and the concentration of GSH were increased. Moreover, mesna did not decrease the anti-tumor property of cisplatin in HePG2 cell lines.
Conclusion
Cisplatin damages the granulosa cells by oxidative stress to deplete the ovarian reserve and mesna could protect ovarian reserve through anti-oxidation. These results might highlight the mechanism of the protection of mesna for ovarian reserve and open an avenue for the application of mesna as a protective additive in cisplatin chemotherapy in clinical practise.
Cancer is a kind of malignant disease with high mortality. With the development of chemotherapy, five-year survival rate and cure rate of cancer patients have been increased steadily [1]. Among the chemotherapeutic agents, cisplatin, with the characteristics of broad-class anti-cancers, strong effects, synergy with multiple chemotherapeutic agents and no cross-resistance, was widely prescribed for a variety of tumors (bladder carcinoma, adrenal cortex carcinoma, breast cancer, lung carcinoma, etc.), either alone or in combination with other agents [2]. However, cisplatin killed cancer cells usually accompanied by damaging normal tissues, especially dividing cells than quiescent cells. Its adverse effects, including neurotoxicity, nephrotoxicity, ototoxicity etc. have been widely announced [3-5]. In recent years, the ovarian damages which were the long-term consequences of exposure to chemotherapies have drawn great attentions in female cancer patients [6-9]. Cisplatin caused ovarian failure with an odd ratio of 1.77 (results from medical records of 168 cancer patients treated by chemotherapies) [1]. Cisplatin induced ovarian dysfunctions such as menstrual disorders, premature menopause, infertility, etc., which resulted in a profound impact on patients' self-esteem and their life quality and also increased medical costs [10-12]. Therefore, maintaining or restoring ovarian functions after chemotherapy has become an important issue for many younger female cancer survivors who were anxious for a normal reproductive life.
Cisplatin is a kind of cell cycle non-specific (CCNS) antineoplastic agents. It was considered that the formation of cisplatin-DNA adducts in the nuclei of tumor cells was responsible for cisplatin's anti-tumor property [13,14], while the cisplatin-induced oxidative stress was a possible mechanism of its toxicities on kidney and internal ear [15-17]. Therefore, anti-oxidants were often used to antagonize the adverse effects caused by cisplatin by virtue of blocking oxidative stress [16-19]. Mesna (2-mercaptoethane sulfonate), a Food and Drug Administration approved anti-oxidant, played an important role in preventing chemotherapeutic agent induced urotoxicity, ototoxicity and intestinal damage in clinic by means of scavenging reactive oxygen species (ROS) and enhancing anti-oxidative state in the tissues [15,20,21]. Recently, it was found that the administration of mesna during low-dose cisplatin treatment protected the ovaries by decreasing the loss of growing follicles in the ovaries [22]. However, it still remains unclear how mesna could protect ovaries from cisplatin induced damage in cancer patients and to which extend mesna could be protective.
Therefore, this present study especially concerned about the anti-oxidation of mesna to remove oxidative stress products generated by cisplatin administration, then protect ovarian reserve. The research results will benefit female cancer patients treated with cisplatin and be helpful for clinical application of chemotherapy protective agents.
Forty eight adult female Sprague-Dawley (SD) rats (weight, 200-250 g age, 65-75 days) were purchased from the Animal Center of Capital Medical University (Beijing, China). Rats were maintained under standard housing conditions with a 12 hours light/dark cycle. Food and water could be obtained ad libitum. All procedures were performed in accordance with the Health Guide for the Care and Use of Laboratory Animals of Capital Medical University and the Institutional Animal Care Committee approved the work.
Rats were randomly divided into 6 groups (8 rats per group): normal saline control (NS), mesna (M, 200 mg/kg), low-dose cisplatin (CL, 4.5 mg/kg), high-dose cisplatin (CH, 6.0 mg/kg). The lethal dose (50) of cisplatin on rats was 7.4 mg/kg [23], low-dose cisplatin plus mesna (CL+M, 4.5 mg/kg+200 mg/kg); high-dose cisplatin plus mesna (CH+M, 6.0 mg/kg+200 mg/kg). Mesna (M1511, Sigma-Aldrich, St. Louis, MO, USA) and cisplatin (479306, Sigma-Aldrich) were diluted in normal saline immediately before use. The rats in NS, M, CL, or CH groups received weekly intraperitoneal injection of normal saline, mesna or cisplatin, respectively, twice in total. In CL+M and CH+M groups, the rats for the first time received mesna injection 30 minutes before the administration of cisplatin. After one week, the rats received mesna and cisplatin for the second time in the same way.
The rats were killed by an overdose of 10% chloral hydrate (5 mL/kg) five days after the second injections. One ovary of each rat was frozen immediately in liquid nitrogen and stored at -80℃ for oxidative stress assay and the other ovary was fixed in 4% paraformaldehyde (0.1 M phosphate buffer, pH 7.3). The fixed specimens were dehydrated and embedded in paraffin for hematoxylin and eosin (H&E) staining and immunofluoresence.
The paraffin embedded ovaries were cut into serial 5 µm sections. Three sections were randomly selected from one ovary for immunofluorescence observations. Immunofluorescence procedure was as follows: paraffin sections were deparaffinized with xylene and rehydrated through a graded series of ethyl alcohol (70-100%) for 5 minutes each. For anti-gen retrieval, the sections were heated under high-pressure with citrate buffer (pH 6.0) and incubated with 1% bovine serum albumin (BSA) for blocking non-specific anti-body binding. The goat anti-Müllerian hormone (AMH C-20: sc-6886, Santa Cruz Biotechnology, Santa Cruz, CA, USA) as primary antibody was diluted to a concentration of 1:100 for incubation overnight at 4℃. After being washed for 5 minutes by 3 times with phosphate buffered saline (PBS), the specimens were incubated with the secondary anti-body, Alexa Fluor 488 donkey anti-goat IgG (1:200, Molecular Probes, Eugene, OR, USA) for 1 hour. After washing, the specimens were mounted with Fluorescent Mounting Medium (Thermo Electron Co., Waltham, MA, USA). Control specimens were prepared in the same manner, but the primary anti-body was omitted. Slides were examined using a fluorescence microscope (Nikon 80i, Tokyo, Japan) with an excitation wavelength of 494 nm.
The analysis of the AMH-positive follicles was based on two factors: the intensity and the distribution of the fluorescent staining [22]. The intensity for each follicle in an ovarian section was graded 0-3 as follows: 0, no staining 1, weak staining 2, moderate staining and 3, strong staining. The distribution was graded as 1 or 2: 1, ≤50% of the structure staining and 2, ≥50% of the structure staining. The total score was obtained from the multiplication of the intensity and the distribution. The score ≥2 was considered positive for AMH. Two independent observers evaluated each slide.
Primordial, primary, preantral and antral follicles were counted on H&E staining slides according to the criteria of Oktay et al. [24].
0.5-1.0 g ovarian tissue homogenate was prepared for the detection of malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione (GSH), respectively. Protein concentration was determined by the bicinchoninic acid (BCA) method with BSA as a reference standard. Assay kits for MDA, SOD, and GSH were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). MDA was measured at a wavelength of 532 nm by reacting with thiobarbituric acid to form a stable chromophoric product. The content of MDA was expressed as nmol/mg protein. Activity of SOD was measured at 550 nm wavelength by nitroblue tetrazolium reduction assay following the reduction of nitrite by a xanthine-xanthine oxidase system, which is a superoxide anion generator. The activity of SOD was expressed as U/mg of protein, where 1 U of the enzyme was defined as the amount of enzyme required to inhibit the rate of epinephrine auto-oxidation by 50% under the conditions of the assay. GSH consumption was determined with 5,5'-dithio-bis-(2-nitrobenzoic acid) DTNB measuring the formation of 5-thio-2-nitrobenzoate at 412 nm wavelength.
Human hepatoma HepG2 cell line, obtained from the American Type Culture Collection (ATCC), were cultured in DMEM (Sigma-Aldrich) supplemented with 10% fetal calf serum (FCS, Invitrogen, Carlsbad, CA, USA). Cells were seeded in a 96 well microplate (Corning, New York, NY, USA) and left for 24 hours at 37℃ in a humidified atmosphere of 5% CO2/95% air. Then the medium was replaced with fresh medium containing different concentrations of cisplatinor cisplatin+mesna: 2.5, 5, 10, 20, 40, or 80 µg/mL. The concentrations of cisplatin and mesna in a cisplatin+mesna well were equal. An equal amount of culture medium was set as control. After incubation for 24, 48, or 72 hours, the cells were treated with methyl thiazolyl tetrazolium (MTT) solution (final concentration of 5 mg/mL) for 4 hours (37℃). The medium was removed and the crystals were dissolved in dimethyl sulfoxide (DMSO, Sigma). The absorbance (A)was measured at 490 nm by a microplate reader (Bio-Rad 680, Bio-Rad Co., Hercules, CA, USA).The viability of HepG2 cells was calculated as: Asample/Acontrol×100%.
Data were from three independent experiments and results were presented as means±standard error of the mean (SE). Statistical analysis was performed with SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance and linear contrast method were used for analysis and p<0.05 was considered as statistically significant.
AMH was expressed in granulosa cells of primary follicles, preantral follicles and small antral follicles in all groups (Fig. 1A). However, AMH was not detected in theca cells, oocytes and interstitial cells.
The quantitative analysis for AMH-positive follicles in the 6 groups was presented in Fig. 1B (H&E staining images not shown). There was no significant difference in AMH-positive follicle count between NS group and M group. The percentage of AMH-positive follicles in CL and CH groups were significantly less than those in NS group and M group, respectively (p<0.05) and significant decrease was detected in CH group when compared with CL group (p<0.05). The percentage of AMH-positive follicles in CL+M group was significantly higher than that in CL group (p<0.05), butthere was no significant difference between CH+M group and CH group (p=0.225).
Effects of cisplatin and mesna on the viability of HepG2 cells were determined by MTT assay. Cisplatin, ranging from 2.5 to 80 µg/mL, significantly inhibited the growth of HepG2 cells in a time- and dose-dependence manner (p<0.05) (Fig. 2). The anti-tumor efficacy was not significantly interfered when cisplatin was co-administered with mesna at different dose and treatment time (p>0.05) (Fig. 2).
MDA is a product of lipid peroxidation. After either low- or high-dose cisplatin administration, ovarian MDA levels were increased significantly, compared with NS group and M group, respectively (p<0.05). With the dose increase of cisplatin, the MDA levels in rat ovaries were significantly increased (p<0.05). MDA levels were significantly decreased when mesna were administered in combination with high-dose cisplatin (p<0.05) (Fig. 3A).
Enzymatic activities of SOD in ovaries were decreased both in CL and CH rats compared with NS group and M group, respectively (p<0.05). With the dose increase of cisplatin, the enzymatic activities of SOD in ovaries were decreased (p<0.05). SOD activities in ovaries were significantly increased when mesna were co-admininstered with either low or high-dose cisplatin (p<0.05) (Fig. 3B).
After either low- or high-dose cisplatin administration, ovarian GSH levels decreased significantly, compared with NS group and M group, respectively (p<0.05). GSH levels in CL+M and CH+M ovaries were significantly higher when compared with CL and CH groups, respectively (p<0.05) (Fig. 3C).
In addition, there was no significant difference in ROS detections between Normal Saline group and single mesna group, suggesting no dramatic toxicity and any other side-effect from mesna.
As granulosa cells in follicles are characterized by rapid turnover like tumor cells, ovaries are extremely sensitive to cytotoxic chemotherapeutic agents which can induce severe gonadal damages. This present study showed that cisplatin damaged ovarian tissues by increasing products of lipid peroxidation and decreasing anti-oxidation capability, which were consistent with the previous literatures [15]. Though the adverse effects of chemotherapy on ovaries were obvious, chemotherapy as an efficient treatment on cancers was still of great medical values. Thus, it is essential to withhold chemotherapeutic adverse effects as much as possible while protect its anti-tumor effects. Our results in this study also showed that mesna could protect ovaries from cisplatin-induced damages by anti-oxidation without inhibiting anti-tumor effects of cisplatin.
Ovarian reserve is a term used to denote the ability of an ovary to be able to produce sufficient numbers of mature oocytes for adequate fertility [25]. It is crucial to assess the ovarian reserve after chemotherapy, which indicates the reproductive potential. The targets of the chemotherapeutic agents could be the granulosa cells that line and support the developing follicles. Any damage to these dividing cells would affect the oocyte maturation and consequently lead to follicular destruction and ovarian dysfunction. AMH, also named Müllerian inhibiting substance, a 140 kDa polypeptide, is a member of TGF-β superfamily [26]. AMH is produced by granulosa cells of small growing (i.e., preantral and small antral) nonatretic follicles in rat and mouse ovaries [26,27]. AMH inhibits recruitment of primordial follicles into the growing pool, while at cyclic recruitment AMH lowers the follicle-stimulating hormone (FSH)-sensitivity of follicles [26,28]. Results obtained from AMH knock-out mice indicated that AMH regulated the development of early follicles in two ways, as a negative stimulator of follicular maturation and as an inhibitor of FSH-sensitivity of growing follicles, serving to negatively modulate the FSH dependent selection of the dominant follicles [29,30]. AMH was stable in fluctuated gonadotropic status and reflected follicle population; therefore, AMH was considered as a reliable marker for the ovarian reserve assessment [31,32] and was often used as an indicator of ovarian damage [33]. Results here showed that the percentage of AMH-positive follicles was significantly decreased when administered with cisplatin (both low and high dose), compared with NS group, suggesting that cisplatin damaged the key ovarian follicles. The decreased AMH facilitated the recruitment of the stable primordial follicles into the growing pool more rapidly and accelerated the growth of the FSH-sensitive follicles, which, accordingly, resulted in more chemotherapeutic-sensitive follicles and ovarian reserve damages [34].
As we know, granulosa cells are the cisplatin target cells in follicles, but what's the underlying molecular mechanism? Might it be as well due to the increased oxidative stress just as that detected in cisplatin-induced injuries in renal tubular cells and cochlear cells [4,35]? DNA was the primary target of cisplatin. The formation of cisplatin-DNA adducts structurally distorted the DNA and restrained the DNA replications, which was established as main events responsible for its anti-tumor property [14]. During the formation of cisplatin-DNA adducts, oxygen free radicals were generated. Overproduction of the oxygen free radicals induced oxidative stress, which was responsible for the cisplatin-related tissue toxicity [14]. Oxygen free radicals affected the cell components such as lipid, protein, DNA and carbohydrates, in which lipids were the most sensitive. Oxygen free radicals enhanced lipid peroxidation. Lipid peroxidation not only changed the fluidity and permeability of biomembranes, but also generated MDA, the stable end product, which caused the cross-linking and polymerization of macromolecules, such as proteins, nucleic acid, etc. Therefore, MDA level represented the lipid peroxidation and the cell oxidative injury. In this study, after either low- or high-dose cisplatin administration, ovarian MDA levels were increased significantly, compared with NS group and the MDA level in CH group was much higher that that in CL group. The increased MDA in cisplatin administered ovaries indicated that cisplatin could induce over lipid peroxidation in ovarian tissues and the end product, MDA, was cytotoxicitive. SOD is one of the most important anti-oxidant enzymes, which catalyzes the conversion of superoxide radicals to hydrogen peroxide, playing a crucial role in the oxidation-anti-oxidation balance. Significant decreases of the SOD activities in cisplatin-administered rat ovaries indicated impairments in the anti-oxidative defenses. GSH, present in cells at high concentrations, represents another major thiol anti-oxidant defense, maintaining the intracellular environment for protecting against both oxidative stress and electrophilic compounds. Significant GSH decreases were observed in ovaries of both low-and high-dose cisplatin-administered rats, indicating cisplatin impaired the non-enzyme anti-oxidant defenses via depleting GSH contents in ovaries. The results above suggested a role of free radicals in cisplatin-induced toxicity in granulasa cells of growing follicles.
Since overproduction of the oxygen free radicals and inadequacy of anti-oxidative defense system lead to the oxidative injuries in ovaries, we hypothesized that scavenging the toxic oxidative metabolites would attenuate the ovarian injuries resulted from oxidative stress. Mesna was used in combination with chemotherapeutic agents to decrease cytotoxicities by scavenging oxygen free radicals via its sulfhydryl group (-SH) [20,36]. In this study, AMH-positive follicles were significantly increased in CL+M group when compared with CL group, suggesting that mesna could protect granulosa cells in growing follicles. However, we didn't observe significant increase of AMH-positive follicles in CH+M group compared with CH group. Considering that the ovarian damages were much more severe in CH group than in CL group, the same dose and administration time of mesna might not be able to reverse the intensive damages. MDA was significantly decreased in CH+M group when compared with CH group. Results also revealed that SOD activities and GSH levels were increased when mesna was administered in combination with either low- or high-dose cisplatin compared with single cisplatin administration. These oxidative stress indexes suggested that mesna could restrain the ovarian lipid peroxidation and GSH depletion induced by cisplatin so as to increase the anti-oxidative abilities to refrain from the oxygen free radical damages. Though mesna dramatically suppressed the oxidative stress and enhanced the anti-oxidative defense both in low- and high-dose cisplatin administrations, we actually did not observe apparent increase of AMH-positive follicle percentage in mesna plus high-dose cisplatin co-administration. We speculated that the repairing oxidative-anti-oxidative balance was not as much as needed to increase significant growing follicles under the condition of high-dose cisplatin induced severe ovarian damage. Thus the future study will be carried out using higher-dose mesna and longer treatment time. Additionally, as the animals used in the current study are normal and of non-tumorigenic model, there would be a discrepancy with cisplatin chemotherapy in clinic. In spite of the limitation in explaining the protective effect of mesna during cisplatin chemotherapy in tumors, we believe that mesna plays a role in the cisplatin-induced ovarian damage to some extent. We also suggest that a tumorigenic animal model should be used in further study to strengthen our results obtained from normal animal in this present experiment.
One considerable issue in the administration of protective addictives during chemotherapies was that the protective addictives do not weaken the chemotherapeutic effects as far as possible. Though the protection of mesna was achieved without attenuating the efficacy of co-administered anti-cancer agents such as ifosfamide and cyclophosphamide [20], there was inconsistence in the effect on platinum chemotherapeutic agents. It was reported that the active thiol monomer of mesna could bind to platinum drugs and reduce their efficacy by a direct reaction with platinum molecule [37,38] while Kangarloo et al. [39] ruled out the influence of mesna on the pharmacokinetics of cisplatin and carboplatin. In the present study, we measured the influence of cisplatin and mesna on the cisplatin sensitive HepG2 cell line either with single administration or co-administration. Result showed that mesna did not markedly suppressed the anti-tumor effects of cisplatin however, we could not completely exclude the influence of mesna on the viability of cisplatin-treated tumor cells. Furthermore, there were still a number of ambiguities concerning that whether mesna did not interfere with the anti-tumor efficacy of cisplatin when used for other cancers besides hepatoma. Therefore, further researches on other cancer cell lines are highly expected.
In conclusion, this present study provided evidences that cisplatin damaged ovarian reserve by increasing oxidative stress and decreasing anti-oxidation capability. Mesna could protect ovarian reserve from cisplatin-induced damages through potent anti-oxidation without obvious inhibition of anti-tumor efficacy of cisplatin.
Figures and Tables
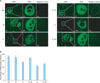 | Fig. 1Representative immunofluorescence images of follicles in normal saline control (NS), mesna (M), low-dose cisplatin (CL), CL+M, high-dose cisplatin (CH), and CH+M groups, showing anti-Müllerian hormone (AMH) expression. I0, no staining; I1, weak staining; I2, moderate staining; I3, strong staining. D1, ≤50% of the structure staining; and D2, ≥50% of the structure staining. (B) Percentage of AMH-positive follicles in NS, M, CL, CL+M, CH, and CH+M groups. p<0.05 for NS vs. CH, NS vs. CL, M vs. CH, C vs. CL, CL vs. CH, and CL vs. CL+M. |
 | Fig. 2Effects of cisplatin or cisplatin+mesna on the viability of HepG2 cells at different dose and treatment time [24 hr (A), 48 hr (B), 72 hr (C)] by methyl thiazolyl tetrazolium assay. *Compared to corresponding control, p<0.05.There is no significant difference in the viability of HepG2 cells between cisplatin group and cisplatin+mesna group. |
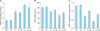 | Fig. 3(A) Malondialdehyde (MDA) level in normal saline control (NS), mesna (M), low-dose cisplatin (CL), CL+M, high-dose cisplatin (CH), and CH+M groups. p<0.05 for NS vs. CH, NS vs. CL, M vs. CH, M vs. CL, CL vs. CH, and CH vs. CH+M. (B) Superoxide dismutase (SOD) activity in NS, M, CL, CL+M, CH and CH+M groups. p<0.05 for NS vs. CH, NS vs. CL, M vs. CH, M vs. CL, CL vs. CH, CL vs. CL+M, and CH vs. CH+M. (C) Glutathione (GSH) level in NS, M, CL, CL+M, CH and CH+M groups. p<0.05 for NS vs. CH, NS vs. CL, M vs. CH, M vs. CL, CL vs. CL+M, and CH vs. CH+M. |
ACKNOWLEDGMENTS
This work was supported inpart by grants (30971349, 81101769 and 81001044) from the National Science Foundation of China (NSFC), grant (PHR201007113) from the Funding Project for Academic Human Resources Development in Institutions of Higher Learning under the Jurisdiction of Beijing Municipality (IHLB) and grant (2012ZR07) from the Science Foundation of Capital Medical University.
References
1. Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol. 2000. 169:123–131.
2. Lebwohl D, Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer. 1998. 34:1522–1534.
3. Berger T, Malayeri R, Doppelbauer A, Krajnik G, Huber H, Auff E, et al. Neurological monitoring of neurotoxicity induced by paclitaxel/cisplatin chemotherapy. Eur J Cancer. 1997. 33:1393–1399.
4. Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Eur J Cancer. 2004. 40:2445–2451.
5. Townsend DM, Tew KD, He L, King JB, Hanigan MH. Role of glutathione S-transferase Pi in cisplatin-induced nephrotoxicity. Biomed Pharmacother. 2009. 63:79–85.
6. Yucebilgin MS, Terek MC, Ozsaran A, Akercan F, Zekioglu O, Isik E, et al. Effect of chemotherapy on primordial follicular reserve of rat: an animal model of premature ovarian failure and infertility. Aust N Z J Obstet Gynaecol. 2004. 44:6–9.
7. Lutchman Singh K, Davies M, Chatterjee R. Fertility in female cancer survivors: pathophysiology, preservation and the role of ovarian reserve testing. Hum Reprod Update. 2005. 11:69–89.
8. Sklar CA, Mertens AC, Mitby P, Whitton J, Stovall M, Kasper C, et al. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2006. 98:890–896.
9. Maltaris T, Seufert R, Fischl F, Schaffrath M, Pollow K, Koelbl H, et al. The effect of cancer treatment on female fertility and strategies for preserving fertility. Eur J Obstet Gynecol Reprod Biol. 2007. 130:148–155.
10. Wallace WH, Shalet SM, Crowne EC, Morris-Jones PH, Gattamaneni HR, Price DA. Gonadal dysfunction due to cis-platinum. Med Pediatr Oncol. 1989. 17:409–413.
11. Stroud JS, Mutch D, Rader J, Powell M, Thaker PH, Grigsby PW. Effects of cancer treatment on ovarian function. Fertil Steril. 2009. 92:417–427.
12. Nasir J, Walton C, Lindow SW, Masson EA. Spontaneous recovery of chemotherapy-induced primary ovarian failure: implications for management. Clin Endocrinol (Oxf). 1997. 46:217–219.
13. Evans DL, Dive C. Effects of cisplatin on the induction of apoptosis in proliferating hepatoma cells and nonproliferating immature thymocytes. Cancer Res. 1993. 53:2133–2139.
14. Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Perez JM. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med Chem. 2007. 7:3–18.
15. Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. 2007. 226:157–167.
16. Choi J, Im GJ, Chang J, Chae SW, Lee SH, Kwon SY, et al. Protective effects of apocynin on cisplatin-induced ototoxicity in an auditory cell line and in zebrafish. J Appl Toxicol. 2013. 33:125–133.
17. Satoh M, Kashihara N, Fujimoto S, Horike H, Tokura T, Namikoshi T, et al. A novel free radical scavenger, edarabone, protects against cisplatin-induced acute renal damage in vitro and in vivo. J Pharmacol Exp Ther. 2003. 305:1183–1190.
18. Antunes LM, Darin JD, Bianchi Nde L. Effects of the antioxidants curcumin or selenium on cisplatin-induced nephrotoxicity and lipid peroxidation in rats. Pharmacol Res. 2001. 43:145–150.
19. Naziroglu M, Karaoglu A, Aksoy AO. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology. 2004. 195:221–230.
20. Siu LL, Moore MJ. Use of mesna to prevent ifosfamide-induced urotoxicity. Support Care Cancer. 1998. 6:144–154.
21. Ypsilantis P, Tentes I, Assimakopoulos SF, Kortsaris A, Scopa CD, Simopoulos C. Mesna ameliorates intestinal mucosa damage after ifosfamide administration in the rabbit at a dose-related manner. J Surg Res. 2004. 121:84–91.
22. Yeh J, Kim BS, Peresie J. Protection against cisplatin-induced ovarian damage by the antioxidant sodium 2-mercaptoethanesulfonate (mesna) in female rats. Am J Obstet Gynecol. 2008. 198:463.e1–463.e7.
23. Dale O, Mortensen B, Thommesen L, Hagen B. Cisplatin toxicity in the rat may be influenced by anaesthetic agents. Acta Anaesthesiol Scand. 2000. 44:770.
24. Oktay K, Schenken RS, Nelson JF. Proliferating cell nuclear antigen marks the initiation of follicular growth in the rat. Biol Reprod. 1995. 53:295–301.
25. Yeh J, Adashi E. Yen S, Jaffe R, Barbieri R, editors. The ovarian life cycle. Reproductive endocrinology. 1999. 4th ed. Philadelphia, PA: Saunders WB;153–190.
26. Stubbs SA, Hardy K, Da Silva-Buttkus P, Stark J, Webber LJ, Flanagan AM, et al. Anti-mullerian hormone protein expression is reduced during the initial stages of follicle development in human polycystic ovaries. J Clin Endocrinol Metab. 2005. 90:5536–5543.
27. Lee MM, Donahoe PK. Mullerian inhibiting substance: a gonadal hormone with multiple functions. Endocr Rev. 1993. 14:152–164.
28. Gruijters MJ, Visser JA, Durlinger AL, Themmen AP. Anti-Mullerian hormone and its role in ovarian function. Mol Cell Endocrinol. 2003. 211:85–90.
29. Visser JA, Themmen AP. Anti-Mullerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005. 234:81–86.
30. Themmen AP. Anti-Mullerian hormone: its role in follicular growth initiation and survival and as an ovarian reserve marker. J Natl Cancer Inst Monogr. 2005. 34:18–21.
31. Streuli I, Fraisse T, Pillet C, Ibecheole V, Bischof P, de Ziegler D. Serum antimüllerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertil Steril. 2008. 90:395–400.
32. Yeh J, Kim B, Liang YJ, Peresie J. Mullerian inhibiting substance as a novel biomarker of cisplatin-induced ovarian damage. Biochem Biophys Res Commun. 2006. 348:337–344.
33. Visser JA, de Jong FH, Laven JS, Themmen AP. Anti-Mullerian hormone: a new marker for ovarian function. Reproduction. 2006. 131:1–9.
34. Rosendahl M, Andersen CY, la Cour Freiesleben N, Juul A, Lossl K, Andersen AN. Dynamics and mechanisms of chemotherapy-induced ovarian follicular depletion in women of fertile age. Fertil Steril. 2010. 94:156–166.
35. Chirino YI, Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol. 2009. 61:223–242.
36. Kabasakal L, Sehirli AO, Cetinel S, Cikler E, Gedik N, Sener G. Mesna (2-mercaptoethane sulfonate) prevents ischemia/reperfusion induced renal oxidative damage in rats. Life Sci. 2004. 75:2329–2340.
37. Hausheer FH, Kanter P, Cao S, Haridas K, Seetharamulu P, Reddy D, et al. Modulation of platinum-induced toxicities and therapeutic index: mechanistic insights and first- and second-generation protecting agents. Semin Oncol. 1998. 25:584–599.
38. Oprea A, Bazzazi H, Kangarloo B, Wolff JE. The kinetics and mechanisms of the reaction of Mesna with cisplatin, oxiplatin and carboplatin. Anticancer Res. 2001. 21:1225–1229.
39. Kangarloo SB, Gangopadhyay SB, Syme RM, Wolff JE, Gluck S. Influence of mesna on the pharmacokinetics of cisplatin and carboplatin in pediatric cancer patients. Med Oncol. 2004. 21:9–20.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download