Abstract
Objective
To investigate the association between reproductive factors and the risk of ovarian cancer among southern Chinese women.
Methods
A hospital-based case-control study was undertaken in Guangzhou, Guangdong Province, between 2006 and 2008. A structured questionnaire was used to obtain information on parity, oral contraceptive use and other reproductive factors in a sample of 500 incident ovarian cancer patients and 500 controls (mean age, 59 years). Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using unconditional logistic regression models.
Results
High parity was inversely associated with ovarian cancer, with an adjusted OR 0.43 (95% CI, 0.30 to 0.62) for women who had given birth to 3 or more children compared to women who had given no more than one birth. Ever use of oral contraceptives was also protective against ovarian cancer; adjusted OR 0.56 (95% CI, 0.40 to 0.78). No association was found for hormone replacement therapy, menopausal status, hysterectomy and family history of ovarian and/or breast cancer.
Ovarian cancer ranks as the seventh most common cause of cancer mortality among women [1], accounting for 4.2% of cancer deaths [2]. It is the eighth most common cancer in women, with approximately 225,000 new cases reported worldwide in 2008 [1]. The incidence of ovarian cancer varies geographically. The age-standardised rate (per 100,000 females) is only 3.8 in China, relatively lower than developed countries such the USA (8.8) and Australia (7.7) [1]. The majority of ovarian malignancies are epithelial in origin [3] and are often diagnosed in the advanced stages of the disease due to the subtle and non-specific symptoms [4]. There is currently no effective screening method available for the detection of this disease [5], which has an overall five-year survival rate of approximately 45% [6].
Numerous studies have linked reproductive factors with ovarian cancer. Long-term oral contraceptive use and higher parity are consistently associated with a reduced ovarian cancer risk [7-19]. Hysterectomy has been suggested to be protective, estimated to confer a 30%-50% risk reduction [20-23]. The evidence regarding use of hormone replacement therapy is somewhat conflicting, with some studies reporting a greater risk of ovarian cancer [19,24], and others revealing no association [9,12,25]. In contrast, increased ovarian cancer risk has been reported for postmenopausal women [13,26,27] and having a family history of breast or ovarian cancer [12,28].
The majority of studies examining the relationship between reproductive factors and ovarian cancer have been conducted in countries with a high incidence of the disease. The aim of this case-control study was to investigate associations between ovarian cancer risk and reproductive factors among southern Chinese women, a low incidence population.
This case-control study was undertaken in Guangzhou, the capital city of Guangdong Province in Southern China, between August 2006 and July 2008. Participants were recruited from four public hospitals, i.e., the Overseas Hospital (affiliated with Jinan University), General Hospital of Guangzhou Military Command, Zhujiang Hospital and Second Affiliated Hospital of Zhongshan University. Cases were incident patients who had been histopathologically diagnosed with an epithelial ovarian tumour within the past 12 months. Half of all case tumours were classified as serous, and borderline malignancy cases were included. Controls were patients who were recruited from wards of the departments of physiotherapy, respiratory disease, gastroenterology, ophthalmology and orthopedics. Subjects were required to be less than 75 years of age, and residents of the metropolitan Guangzhou area for at least the past ten years.
Potential cases were identified from the daily census of the hospitals. To ensure complete ascertainment of cases, all hospital medical records and laboratory pathology reports were reviewed. Pathological diagnoses were derived from the International Histological Classification of Ovarian Tumors recommended by the International Federation of Gynecology and Obstetrics [29]. Patients were excluded when ovarian cancer was histopathologically confirmed to be neither the primary nor final diagnosis, or if they reported memory problems affecting their recall of past events. Of the total 504 ovarian cancer cases consecutively recruited from the four hospitals, 500 patients consented to participate and were capable of being interviewed.
During the same period, 512 eligible controls were identified and frequency matched to cases by age (±5 years). The following exclusion criteria applied for the controls: 1) previous diagnosis of ovarian cancer or other malignant diseases; 2) a history of bilateral oophorectomy; 3) memory problems; 4) on long-term mobility restriction; in addition to advanced age (i.e., exceeding 75 years) and non-residency. Whenever more control subjects appeared to be available than could be interviewed, a selection of ward and patient identification was made using random numbers. After initial screening of potential controls using the hospital daily census records, all eligible inpatients had their diagnosis confirmed by histopathological reports to avoid misclassification of the case-control status. This systematic selection process was implemented throughout the recruitment period. Twelve women who did not satisfy the eligibility conditions or declined the interview were later excluded, resulting in a final sample of 500 controls available for analysis.
An appointment for a 45 minute face-to-face interview with each participant was arranged with the assistance of nursing staff, to avoid interference with treatment at the ward and before being discharged from hospital. All participants provided formal consent prior to the interview. They were also assured of confidentiality and their right to withdraw without prejudice. Participants were interviewed in the presence of their next-of-kin whenever possible, to minimize recall error. The interviews were conducted in either Mandarin or the Cantonese dialect. All participants were blinded to the study hypothesis. The project protocol was approved by the participating hospitals, the doctors-in-charge of the relevant wards, and the Human Research Ethics Committee of Curtin University (approval number HR 78/2006).
A structured questionnaire used in a previous study in Hangzhou, China [27], was administered to obtain demographic and lifestyle characteristics, including age, weight (kg), height (m), location of residence, employment status, education level, smoking status, alcohol consumption and marital status. Detailed information on reproductive history, hormonal status and heredity was obtained. Self-reported data were verified against corresponding entries in medical records and any discrepancy found was subsequently rectified.
The sample characteristics of cases and controls were summarised using descriptive statistics. Univariate analysis was then undertaken to compare cases and controls in terms of reproductive factors. Odds ratios (ORs) and associated 95% confidence intervals (CIs) from unconditional logistic regression models were used to ascertain the association between reproductive factors and the risk of ovarian cancer. Only women who had at least one live birth were included in the multivariate analysis for parity, due to the low number of nulliparous women. For ease of analysis, oral contraceptive duration (months) was divided into approximate tertiles based on the distribution of controls, with the lowest tertile used as the reference category.
Tests for linear trend were also conducted for the corresponding continuous variables in the logistic regression models to assess the dose-response relationship between reproductive factors and the ovarian cancer risk. The following established or plausible confounding factors were adjusted for in the logistic regression models: age at interview (years), smoking status (never, ever), alcohol drinking (no, yes), education level (none/primary, secondary, vocational/tertiary) and body mass index (5 years ago, kg/m2). In addition, mutual adjustment was made for parity (≤1, ≥2), oral contraceptive use (never, ever), hormone replacement therapy (no, yes), menopausal status (pre, post), hysterectomy (no, yes) and family history of ovarian and/or breast cancer (no, yes). All statistical analyses were performed using the SPSS ver. 20 (IBM Co., Armonk, NY, USA).
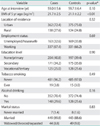
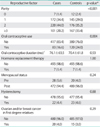
The sample characteristics of women with and without ovarian cancer are shown in Table 1. Compared to controls, women with ovarian cancer had a significantly higher mean body mass index. The two groups appeared to be similar with respect to all other demographic and lifestyle factors. Table 2 provides a comparison of the reproductive factors by case-control status. A significant difference was observed for parity, with one third of controls given birth to 3 or more children, compared to 20% for cases. Ever use of oral contraceptives also differed significantly between groups, with fewer cases ever using oral contraceptives.
The logistic regression results for the reproductive factors are summarised in Table 3. A strong inverse association was evident between parity and ovarian cancer, with a 60% risk reduction among women who had given birth to 3 or more children, compared to women with parity ≤1 (adjusted OR 0.43; 95% CI, 0.30 to 0.62). The corresponding dose-response relationship was also significant (p<0.001). The adjusted OR of ever use of oral contraceptives was 0.56 (95% CI, 0.40 to 0.78), however the effect was not significant for oral contraceptive duration of usage. Hormone replacement therapy, menopausal status, hysterectomy and family history of ovarian and/or breast cancer were not significantly associated with the ovarian cancer risk.
In this study, we found that ever use of oral contraceptives is associated with a reduced risk of ovarian cancer among southern Chinese women. This finding aligns with our previous study in Hangzhou [27] and supports the results of studies with women from higher incidence populations [13,18,26]. A similar protective effect was reported in a meta-analysis of 45 cohort and case-control studies from 21 countries, which found an overall relative risk of 0.73 (95% CI, 0.70 to 0.76) for ever users of oral contraceptives compared to never users [30]. A protective effect for oral contraceptive duration was not observed in this study. Previous research conducted in China similarly reported no association for oral contraceptive duration [31,32]. However, a recent review found that the estimated relative risk of ovarian cancer could decrease by around 20% for every 5 years use of hormonal contraception, and around 50% for 15 years of use [33]. Duration of oral contraceptive use among our study population may be insufficient to produce a protective effect, as only 32% of ever users consumed oral contraceptives for over 5 years. Increasing parity was also inversely associated with the ovarian cancer risk, consistent with several epidemiological studies [7-9,14,16,17,19] and a pooled analysis of 10 case-control studies from the United States [34].
The incessant ovulation hypothesis and the gonadotropin hypothesis are two well-known theories explaining the possible biological mechanisms behind ovarian cancer development. The former suggests that repeated turnover of surface ovarian epithelium occurring in ovulation increases the odds of spontaneous genetic mutations and hence increases the risk of ovarian cancer [35,36]. In the latter hypothesis, elevated gonadotropin levels cause ovarian epithelial cells to become trapped within the surrounding connective tissue, which may lead to the formation of inclusion cysts [37-39]. Oral contraceptive use and higher parity are thought to reduce ovarian cancer risk by decreasing gonadotropin levels and suppressing ovulation [40].
Consideration must be given to the strengths and limitations of this study when interpreting the findings. The implementation of a standardized identification procedure ensured that ascertainment of cases was maximized and complete. To avoid misclassification of the case-control status, only incident patients who had been histopathologically diagnosed with ovarian cancer within the past 12 months were recruited, and all controls were confirmed. A high response rate (98%) was achieved in the recruitment of inpatients through support from the medical doctors and nursing staff. All interviews followed the same procedure for both case and control groups, while recruitment bias was minimized by sampling from different hospitals. Despite the low refusal rate, selection bias was unavoidable because all participants were voluntary and the hospital-based controls were not randomly selected from the community. Nevertheless, the four participating hospitals serve the entire catchment region so that our participants were still representative of the target population. Logistic regression analyses included adjustment for potential confounding variables such as body mass index. Nevertheless, residual confounding may still exist.
In conclusion, high parity and oral contraceptive use were found to reduce ovarian cancer risk in this population of southern Chinese women. Because diagnosis of ovarian cancer occurs usually in the late stages, the findings are important for cancer prevention in this low incidence population.
Figures and Tables
Table 3
Ovarian cancer risk according to reproductive factors in southern Chinese women

CI, confidence interval; OR, odds ratio.
*Estimates from logistic regression models include terms for age (yr), smoking status (never, ever), alcohol drinking (no, yes), education (none/primary, secondary, vocational/tertiary), body mass index (5 yr ago, kg/m2), and mutually adjusted for parity (≤1, ≥2), oral contraceptive use (never, ever), hormone replacement therapy (no, yes), menopausal status (pre, post), hysterectomy (no, yes) and family history of ovarian and/or breast cancer (no, yes). †Ever users of oral contraceptives.
ACKNOWLEDGMENTS
We gratefully acknowledge the willing cooperation given by the patients and medical and nursing staff from the participating hospitals.
References
1. Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin D. Cancer incidence and mortality worldwide. 2010. Lyon: International Agency for Research on Cancer.
2. Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006. 20:207–225.
3. Cho KR, Shih IeM. Ovarian cancer. Annu Rev Pathol. 2009. 4:287–313.
4. Lutz AM, Willmann JK, Drescher CW, Ray P, Cochran FV, Urban N, et al. Early diagnosis of ovarian carcinoma: is a solution in sight? Radiology. 2011. 259:329–345.
5. Freedman J. Ovarian cancer: current and emerging trends in detection and treatment. 2009. New York: Rosen Publishing Group.
6. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012. 62:10–29.
7. Le DC, Kubo T, Fujino Y, Sokal DC, Vach TH, Pham TM, et al. Reproductive factors in relation to ovarian cancer: a case-control study in Northern Vietnam. Contraception. 2012. 86:494–499.
8. Tsilidis KK, Allen NE, Key TJ, Dossus L, Lukanova A, Bakken K, et al. Oral contraceptive use and reproductive factors and risk of ovarian cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer. 2011. 105:1436–1442.
9. Braem MG, Onland-Moret NC, van den Brandt PA, Goldbohm RA, Peeters PH, Kruitwagen RF, et al. Reproductive and hormonal factors in association with ovarian cancer in the Netherlands cohort study. Am J Epidemiol. 2010. 172:1181–1189.
10. Tworoger SS, Fairfield KM, Colditz GA, Rosner BA, Hankinson SE. Association of oral contraceptive use, other contraceptive methods, and infertility with ovarian cancer risk. Am J Epidemiol. 2007. 166:894–901.
11. Modugno F, Ness RB, Allen GO, Schildkraut JM, Davis FG, Goodman MT. Oral contraceptive use, reproductive history, and risk of epithelial ovarian cancer in women with and without endometriosis. Am J Obstet Gynecol. 2004. 191:733–740.
12. Pike MC, Pearce CL, Peters R, Cozen W, Wan P, Wu AH. Hormonal factors and the risk of invasive ovarian cancer: a population-based case-control study. Fertil Steril. 2004. 82:186–195.
13. Tung KH, Goodman MT, Wu AH, McDuffie K, Wilkens LR, Kolonel LN, et al. Reproductive factors and epithelial ovarian cancer risk by histologic type: a multiethnic case-control study. Am J Epidemiol. 2003. 158:629–638.
14. Riman T, Dickman PW, Nilsson S, Correia N, Nordlinder H, Magnusson CM, et al. Risk factors for invasive epithelial ovarian cancer: results from a Swedish case-control study. Am J Epidemiol. 2002. 156:363–373.
15. Bosetti C, Negri E, Trichopoulos D, Franceschi S, Beral V, Tzonou A, et al. Long-term effects of oral contraceptives on ovarian cancer risk. Int J Cancer. 2002. 102:262–265.
16. Titus-Ernstoff L, Perez K, Cramer DW, Harlow BL, Baron JA, Greenberg ER. Menstrual and reproductive factors in relation to ovarian cancer risk. Br J Cancer. 2001. 84:714–721.
17. Chiaffarino F, Pelucchi C, Parazzini F, Negri E, Franceschi S, Talamini R, et al. Reproductive and hormonal factors and ovarian cancer. Ann Oncol. 2001. 12:337–341.
18. Ness RB, Grisso JA, Klapper J, Schlesselman JJ, Silberzweig S, Vergona R, et al. Risk of ovarian cancer in relation to estrogen and progestin dose and use characteristics of oral contraceptives. SHARE Study Group. Steroid Hormones and Reproductions. Am J Epidemiol. 2000. 152:233–241.
19. Tavani A, Ricci E, La Vecchia C, Surace M, Benzi G, Parazzini F, et al. Influence of menstrual and reproductive factors on ovarian cancer risk in women with and without family history of breast or ovarian cancer. Int J Epidemiol. 2000. 29:799–802.
20. Green A, Purdie D, Bain C, Siskind V, Russell P, Quinn M, et al. Tubal sterilisation, hysterectomy and decreased risk of ovarian cancer. Survey of Women's Health Study Group. Int J Cancer. 1997. 71:948–951.
21. Hankinson SE, Hunter DJ, Colditz GA, Willett WC, Stampfer MJ, Rosner B, et al. Tubal ligation, hysterectomy, and risk of ovarian cancer. A prospective study. JAMA. 1993. 270:2813–2818.
22. Chiaffarino F, Parazzini F, Decarli A, Franceschi S, Talamini R, Montella M, et al. Hysterectomy with or without unilateral oophorectomy and risk of ovarian cancer. Gynecol Oncol. 2005. 97:318–322.
23. Parazzini F, Negri E, La Vecchia C, Luchini L, Mezzopane R. Hysterectomy, oophorectomy, and subsequent ovarian cancer risk. Obstet Gynecol. 1993. 81:363–366.
24. Riman T, Dickman PW, Nilsson S, Correia N, Nordlinder H, Magnusson CM, et al. Hormone replacement therapy and the risk of invasive epithelial ovarian cancer in Swedish women. J Natl Cancer Inst. 2002. 94:497–504.
25. Sit AS, Modugno F, Weissfeld JL, Berga SL, Ness RB. Hormone replacement therapy formulations and risk of epithelial ovarian carcinoma. Gynecol Oncol. 2002. 86:118–123.
26. Modugno F, Ness RB, Wheeler JE. Reproductive risk factors for epithelial ovarian cancer according to histologic type and invasiveness. Ann Epidemiol. 2001. 11:568–574.
27. Zhang M, Lee AH, Binns CW. Reproductive and dietary risk factors for epithelial ovarian cancer in China. Gynecol Oncol. 2004. 92:320–326.
28. Parazzini F, Negri E, La Vecchia C, Restelli C, Franceschi S. Family history of reproductive cancers and ovarian cancer risk: an Italian case-control study. Am J Epidemiol. 1992. 135:35–40.
29. Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, et al. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006. 95:Suppl 1. S161–S192.
30. Collaborative Group on Epidemiological Studies of Ovarian Cancer. Beral V, Doll R, Hermon C, Peto R, Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008. 371:303–314.
31. Chen Y, Wu PC, Lang JH, Ge WJ, Hartge P, Brinton LA. Risk factors for epithelial ovarian cancer in Beijing, China. Int J Epidemiol. 1992. 21:23–29.
32. Shu XO, Brinton LA, Gao YT, Yuan JM. Population-based case-control study of ovarian cancer in Shanghai. Cancer Res. 1989. 49:3670–3674.
33. Grimbizis GF, Tarlatzis BC. The use of hormonal contraception and its protective role against endometrial and ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2010. 24:29–38.
34. Kurian AW, Balise RR, McGuire V, Whittemore AS. Histologic types of epithelial ovarian cancer: have they different risk factors? Gynecol Oncol. 2005. 96:520–530.
35. Fathalla MF. Incessant ovulation--a factor in ovarian neoplasia? Lancet. 1971. 2:163.
36. World Cancer Research Fund. American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. 2007. Washington, DC: American Institute for Cancer Research.
37. Cramer DW, Welch WR. Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. J Natl Cancer Inst. 1983. 71:717–721.
38. Zheng H, Kavanagh JJ, Hu W, Liao Q, Fu S. Hormonal therapy in ovarian cancer. Int J Gynecol Cancer. 2007. 17:325–338.
39. Hanna L, Adams M. Prevention of ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2006. 20:339–362.
40. Siskind V, Green A, Bain C, Purdie D. Beyond ovulation: oral contraceptives and epithelial ovarian cancer. Epidemiology. 2000. 11:106–110.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download