Abstract
Objective
To compare the efficacy of the levonorgestrel-releasing intrauterine system (LNG-IUS) and oral norethisterone acetate (NET) for treatment of non-atypical endometrial hyperplasia in perimenopausal women.
Methods
One hundred and twenty perimenopausal women with non-atypical endometrial hyperplasia were selected in this randomized controlled trial. Patients received LNG-IUS (n=59) or NET (n=61; 15 mg/day for 3 weeks/cycle) for 3-6 months. Outpatient follow-up with endometrial biopsies were undertaken at 3, 6, and 12 months intervals after treatment. Outcome measures were; the regression rate, the time to regression and hysterectomy rate.
Results
A significantly higher regression rate was noted in the LNG-IUS group than in NET group at the 3rd, 6th and 12th month follow-up visits using intention-to-treat analysis (67.8% vs. 47.5%, relative risk [RR], 1.42; 79.7% vs. 60.7%, RR, 1.31; and 88.1% vs. 55.7%, RR, 1.58, respectively). However, no significant difference was found regarding the median time to regression (3 months). The hysterectomy rate during the follow-up period was significantly higher in the NET group (57.4% vs.22%, p<0.001).
Endometrial hyperplasia (EH) is a frequently encountered clinical entity peaking in the early 50s and 60s [1,2]. It presents commonly with abnormal uterine bleeding (AUB) [3,4]. However, its clinical importance largely relates to the risk of progression to endometrial carcinoma which is low for simple or complex non-atypical EH (<5%) compared with atypical EH (approximately 30%) [5,6]. Additionally, a 17%-42.6% risk of concurrent endometrial carcinoma was reported in women with a biopsy diagnosis of atypical EH [7,8].
The optimal management of EH is a subject of considerable debate [4,9]. In presence of atypia, fertility preservation with progestin therapy has been attempted in young motivated patients [10-12], however recommendation of hysterectomy seems appropriate [1,4,9-13]. Meanwhile, EH without atypia have been traditionally treated with high-dose progestin therapy [1,4,13,14]. However, its efficacy is often limited by significant side effects e.g. weight gain, edema and irritability [1,4,9]. Additionally, a resistance rate of 14% and a recurrence rate of 6% were reported following cessation of therapy in cases of EH without atypia [15].
Recently, levonorgestrel-releasing intrauterine system (LNG-IUS) has been reported as a successful treatment option for EH [16,17]. Achievement of higher progestin concentrations in the endometrium by many folds compared with oral administration was reported [18]. Accordingly, these higher local concentrations might end up into more consistent results with lower recurrence rates than temporary treatment with oral progestins.
To the best of our knowledge, no randomized controlled trials (RCTs) compared the efficacy of the LNG-IUS and norethisterone acetate (NET) for treatment of non-atypical EH. To examine this issue, we compared both modalities in a prospective RCT in perimenopausal women.
This RCT enrolled women complaining of AUB among those attending the Outpatient Clinic in Mansoura University Hospitals, Egypt in the period from May 2009 to November 2011. A detailed history, examinations and ultrasound evaluation were carried out. Endometrial biopsy samples were obtained by combination of hysteroscopy with dilatation and curettage (D&C) following inpatient admission. Inclusion criteria were those with histologically confirmed non-atypical simple or complex EH, age between 40 and 50 years with an ongoing menstrual cycle for at least 6 months before the onset of AUB and no contraindication to either LNG-IUS or NET e.g., current or a history of deep venous thrombosis, active thrombophlebitis, thromboembolic disorder, or cerebrovascular accident; myocardial infarction or ischemic heart disease and liver disease.
Exclusion criteria were EH with atypia, age >50 years, other pathology e.g., submucosal myomas or polyps, adnexal abnormality, genital infection, hormone therapy or any medication which might affect the menstrual blood loss within the previous 6 months e.g., steroid hormones or anticoagulants, previous endometrial ablation, diabetic and/or hypertensive patients and those unwilling for medical management. The study was approved by Mansoura University Hospital Research Ethics Committee and all participants gave informed consent before inclusion in the trial. The study protocol was registered at the ClinicalTrials.gov (ID: NCT01499602). The trial is reported and analyzed following the Consolidated Standards of Reporting Trials (CONSORT) criteria.
Women were randomized according to a computer-generated random numeric table prepared by an independent statistician with concealment of treatment allocation by use of sealed opaque envelopes that were given to a third party (nurse) who assigned patients to study arms; group A (LNG-IUS) or group B (NET). Outcome assessors i.e., those performing histological diagnosis (two independent gynecological pathologists) and statistical analysis were blinded to the treatment groups.
In group A, LNG-IUS (Mirena, Bayer Schering Pharma Oy, Turku, Finland) was inserted and all women underwent follow-up at regular intervals for one year. During follow-up visits, clinic review, transvaginal ultrasonography (TVS) and endometrial histological surveillance by outpatient Pipelle sampling were carried out. The first two visits were at the 3rd month and 6th month after insertion. Successful treatment was defined as histological regression evident by glandular atrophy and stroma decidualization [19,20]. If complete regression was achieved within 6 months, the follow-up interval was extended to 6 months thereafter. For patients with unsuccessful treatment after six-months, histological surveillance after another 3 and 6 months interval was carried out.
In group B, NET tablets (Cidolut Nor, Chemical Industries Development, Cairo, Egypt) were prescribed at a dose of 5 mg three times daily (15 mg/day) for 3 weeks over three months. Clinical review, TVS and endometrial histological surveillance by outpatient Pipelle sampling was carried out by the end of the 3rd treatment cycle between the 20th and 23rd day of this artificially created menstrual cycle. Successful treatment was defined as mentioned above [19,20]. Women with successful treatment discontinued progestin therapy and were called for follow-up with TVS and outpatient Pipelle sampling 3 months later. Meanwhile, women with persistant EH after 3 months were prescribed NET for another 3 months and then reevaluated at the end of the 6th month. After that patients were counseled for hysterectomy or further 3 months treatment if there was no histological evidence of complete regression. Meanwhile, women with successful treatment discontinued progestin therapy and were called for follow-up 6 months later.
The primary outcome was the proportion of women with complete regression of EH. Secondary outcome measures were; the time to complete regression during the 12 months follow-up period and the rate of hysterectomy. Sample size was calculated based on an expected regression rate of 75%, in cases of non-atypical EH after at least 3 months of any progestin therapy, with at least 3 months follow-up [14]. Accordingly, a total of 98 women was required to show an observed difference of 20% in the regression rate between treatments, with a power of 80% using a two tailed χ2 test with a 5% significance level (type I error). With an assumed attrition rate of 10%, a total of 108 patients were needed (54 in each arm).
Data obtained were statistically analyzed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). Means were compared using the unpaired Student's t-test while proportions were compared using the χ2 test and relative risk with 95% confidence interval (CI) were calculated. A p-value of less than 0.05 was considered statistically significant.
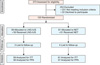
A total of 373 women were assessed for eligibility. One hundred and twenty women were randomly assigned to treatment and comprised the intention-to-treat population. Women received either LNG-IUS (group A, n=59) or NET (group B, n=61). Seven patients with confirmed regression of EH (3 in group A and 4 in group B) were lost to follow up at the end of the 12 months. Fig. 1 shows the flow of participants in the trial. During treatment, 6 patients (9.8%) in group B suffered from some side effects including nausea in 3 patients and weight gain in the other 3 patients, but they continued therapy. There were no significant differences between both groups as regards baseline characteristics, clinical presentation and histological types of EH (Table 1).
Table 2 summarizes the effect of therapy in both groups. Following treatment, a significantly higher regression rate was noted in the LNG-IUS group than in NET group at the 3rd and the 6th month follow-up visits (67.8% vs. 47.5%, RR=1.42 [1.04-1.96], p=0.025; 79.7% vs. 60.7%, RR=1.31 [1.03-1.67], p=0.023, respectively). Another 3 women in group A achieved regression at the 9th month follow up and another 2 at 12 months. The remaining seven patients with unsuccessful treatment at 12 months were counseled for hysterectomy. Histological reports of their hysterectomy specimens showed persistent non-atypical complex EH. On the other hand, the remaining 24 women in group B with confirmed persistent non-atypical complex EH at the 6th month follow up visit were counseled for hysterectomy and the same pathology was shown in histological reports of their hysterectomy specimens. Importantly, at the 12th month follow up visit, a significantly higher regression rate was noted in the LNG-IUS group than in NET group using intention-to-treat analysis (ITT) or the per protocol analysis (88.1% vs. 55.7%, RR=1.58 [1.24-2.01], p<0.001; 92.9% vs. 59.6%, RR=1.55 [1.24-1.95], p<0.001, respectively). However, there was no significant difference between both groups regarding the median time to regression (3 months for each; p=0.697).
During the study, 6 patients in group A requested hysterectomy to be done due to persistent bleeding with the LNG-IUS in situ for about 10 months. Their final histological reports of hysterectomy specimens showed regression of EH. On the other hand, in group B, none of the 29 cases who achieved regression at 3 months showed recurrent hyperplasia in their second follow up visit. However, 3 of them requested hysterectomy to be done due to persistent irregular bleeding and histological assessment of their hysterectomy specimens confirmed regression of EH. Another 8 out of 30 women in group B who attended the 12th month follow up visit requested hysterectomy to be done due to recurrent episodes of AUB. Endometrial regression was reported upon histological evaluation of the hysterectomy specimens. As a total, so far 13/59 patients (22%) in the LNG-IUS group and 35/61 (57.4%) in the NET treatment group have undergone hysterectomy with highly significant difference; RR=0.38 (0.23-0.65), p<0.001. Moreover, the hysterectomy rate in patients who achieved regression in the LNG-IUS group was significantly less than that in the NET treatment (11.5% vs. 29.7%, RR=0.39 [0.16-0.95] respectively, p=0.031) (Table 2). The operation was extrafascial total abdominal hysterectomy (and bilateral salpingo-oophorectomy in women above 45 years) and no atypia or cancer was reported in the hysterectomy specimens.
In this RCT, we have evaluated the efficacy of LNG-IUS in treatment of non-atypical EH in perimenopausal women by comparing it with oral NET. Perimenopausal women were selected as they represent the great sector of outpatient visits presented with AUB necessitating further evaluation and management. NET was used because medroxyprogesterone acetate and megestrol acetate, which represent the most commonly used progestins, are not available at the Egyptian market. Horn et al. [21], treated pre- and perimenopausal patients with complex and atypical EH with NET (5 mg/day) or medroxyprogesterone acetate (10 mg/day) for 3-5 months with an overall remission rate of 61.5%. Bese et al. [22] reported that 3 months of cyclic NET (15 mg/day) treatment reduced both proliferative and apoptotic activities in endometrial tissue with simple non-atypical EH. At the 12th month follow-up visit, a significantly higher regression rate was noted in the LNG-IUS group than in NET group using ITT (88.1% vs. 55.7%). Our findings match those of others who found that 87.5% of patients with non-atypical EH achieved regression by 12 months [17,23]. Furthermore, a recent meta-analysis of 24 observational studies including 1,001 women showed that oral progestins achieved a significantly lower pooled regression rate compared with LNG-IUS for non-atypical complex EH (66% vs. 92%) [16].
In our study, the regression time with the Mirena IUS ranged from 3 to 12 months (median, 3 months) with a significant proportion of the patients (67.8%) achieving regression at the 3rd month. This success rate matches with 66% regression rate within 3 months of Mirena application reported by others with a mean duration of 4.5 months [24]. Different regression times were reported in other studies as follows; range, 4 to 12 months (median, 6 months) [17]; range, 7 to 11.7 months (mean, 9.4 months) [23]. In our study, EH regression rate of 67.8% found after 6 months of Mirena IUS, meanwhile another study [25] reported a 100% regression rate after 6 months. Actually, dissimilarity in the patients' characteristics and other methodical details might explain the differences in achieved regression rate per time in these studies. However, it is noteworthy that beneficial effects were observed within 1 year of therapy in different studies [17,23,25,26] which could be related to complete down-regulation of progesterone receptors in glands coinciding with modulation of apoptosis [27].
During the study, 6 patients requested a hysterectomy due to persistent bleeding with the LNG-IUS in situ for about 10 months. Their final histology result confirmed regression of EH. This finding matches with that reported by others with 2/28 of their patients with non-atypical complex EH requested a hysterectomy to be done due to persistent bleeding with the LNG-IUS in situ for 4 months and 7 months respectively [17]. Importantly, a common side effect of the LNG-IUS is persistent bleeding which may be experienced by up to 35% of its users during the first three treatment months, decreasing to 4% later on [28-30]. This bleeding adds difficulty for reliance on patient symptoms to monitor response in case of EH.
The hysterectomy rate during the 12 months follow-up period in our study was significantly higher in the NET group than LNG-IUS group (57.4% vs. 22%). Moreover, the hysterectomy rate in patients with regression was significantly less in the LNG-IUS group compared with NET (11.5% vs. 29.7%). This finding supports the view that the future use of LNG-IUS to treat non-atypical EH can reduce the number of potentially unnecessary hysterectomies [16,17,23]. No atypia or cancer was found in the hysterectomy specimens. This may be attributed to the accuracy of the initial diagnosis of EH which was carried out by combined hysteroscopy with D&C. In conjunction with targeted biopsies or D&C, hysteroscopy was reported to have an excellent sensitivity, specificity, positive, and negative predictive values of 98%, 95%, 96%, and 98%, respectively to diagnose intrauterine pathology when compared with the histological findings of hysterectomy specimens [13,31].
There are some concerns regarding our study. First, it could be argued that conservative management for EH should be limited to young patients who want to preserve their fertility or patients with medical co-morbidities for whom surgery is contraindicated regardless of atypia. However, a recent survey carried out at the UK pointed out that the majority of the UK gynecologists (52.6%) would prefer two conservative choices (oral progestins or LNG-IUS) before deciding a hysterectomy for non-atypical EH. On the other hand, for atypical EH, the majority of them (83.2%) would perform a hysterectomy and would only consider LNG-IUS or oral progestins as a second or third option in women who wish to retain fertility [32]. A second argument is that endometrial curettage performed for initial diagnosis may have a therapeutic effect by removing the hyperplasia lesion from endometrial cavity; thereby the results of comparison in this study might be influenced. However, although curettage was used for removal of endometrial polyps [33], yet the evidence for a possible therapeutic effect in case of EH is lacking. One study evaluated the use of therapeutic curettage for menorrhagia and showed just a temporary effect as menstrual blood loss was reduced for only 1 month after curettage but then returned to previous levels [34].
Thirdly, this study was not triple-blinded because of the different nature of treatments. However, outcome assessors i.e., those performing the histological diagnosis of specimens and statistical analysis were blinded to the treatment groups. A fourth argument is that with the low progression rate of non-atypical EH into endometrial cancer (<5%) [5,6], hysterectomy is not necessary for those women who do not respond to progestin therapy in 6 months and consideration could be given to continued treatment for a longer time. However, following counseling, those women opted for hysterectomy rather than continued oral progestin therapy. Lastly, follow-up evaluation was performed in our study by Pipelle endometrial sampling. Concerns were raised regarding its accuracy as compared with D&C in diagnosing EH. A recent trial evaluated this issue among 673 patients [35]. Notably higher agreements were found in patients having EH with and without atypia. All cases (100%) of non-atypical EH and 90% of atypical EH which were diagnosed by D&C were detected on Pipelle biopsy. Sensitivity of Pipelle biopsy in detection of non-atypical EH was 67% vs. 62% for D&C and 75% for atypical EH vs. 83% for D&C [35]. Another concern is that the presence of the LNG-IUS in the uterine cavity may affect the accuracy of the Pipelle endometrial biopsy obtained during the follow up period. Of note, a prospective multicenter Korean study is currently under-running to estimate the treatment efficacy of LNG-IUS for EH as well as evaluating the consistency of the results of the office endometrial aspiration biopsy performed with the LNG-IUS in situ compared with that obtained by D&C after LNG-IUS removal [36]. Despite the above-mentioned concerns, the strength of our study resides in being the first RCT to date that evaluated the efficacy of the LNG-IUS for treatment of non-atypical EH.
In conclusion, our study illustrates the superiority of LNG-IUS than the high dose oral NET therapy for non-atypical EH and should be regarded as a valuable treatment option in these cases being simple, highly effective within one year of treatment in addition to its superior compliance. Moreover, its use can reduce the number of potentially unnecessary hysterectomies performed in this subgroup and hence reduce morbidity and health care costs.
Figures and Tables
Fig. 1
CONSORT flow chart of participants in this trial. ITT, intention-to-treat analysis; LNG-IUS, levonorgestrel-releasing intrauterine system; NET, norethisterone acetate; PPA, per protocol analysis.
References
1. Gultekin M, Dogan NU, Aksan G, Ozgul N. Management of endometrial hyperplasia. Minerva Ginecol. 2010. 62:433–445.
2. Reed SD, Newton KM, Clinton WL, Epplein M, Garcia R, Allison K, et al. Incidence of endometrial hyperplasia. Am J Obstet Gynecol. 2009. 200:678.e1–678.e6.
3. Anastasiadis PG, Skaphida PG, Koutlaki NG, Galazios GC, Tsikouras PN, Liberis VA. Descriptive epidemiology of endometrial hyperplasia in patients with abnormal uterine bleeding. Eur J Gynaecol Oncol. 2000. 21:131–134.
4. Espindola D, Kennedy KA, Fischer EG. Management of abnormal uterine bleeding and the pathology of endometrial hyperplasia. Obstet Gynecol Clin North Am. 2007. 34:717–737.
5. Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia: a long-term study of "untreated" hyperplasia in 170 patients. Cancer. 1985. 56:403–412.
6. Lacey JV Jr, Chia VM. Endometrial hyperplasia and the risk of progression to carcinoma. Maturitas. 2009. 63:39–44.
7. Trimble CL, Kauderer J, Zaino R, Silverberg S, Lim PC, Burke JJ 2nd, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006. 106:812–819.
8. Eddib A, Allaf B, Lee J, Yeh J. Risk for advanced-stage endometrial cancer in surgical specimens from patients with complex endometrial hyperplasia with atypia. Gynecol Obstet Invest. 2012. 73:38–42.
9. Marsden DE, Hacker NF. Optimal management of endometrial hyperplasia. Best Pract Res Clin Obstet Gynaecol. 2001. 15:393–405.
10. Koskas M, Azria E, Walker F, Luton D, Madelenat P, Yazbeck C. Progestin treatment of atypical hyperplasia and well-differentiated adenocarcinoma of the endometrium to preserve fertility. Anticancer Res. 2012. 32:1037–1043.
11. Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol. 2012. 125:477–482.
12. Trimble CL, Method M, Leitao M, Lu K, Ioffe O, Hampton M, et al. Management of endometrial precancers. Obstet Gynecol. 2012. 120:1160–1175.
13. Montgomery BE, Daum GS, Dunton CJ. Endometrial hyperplasia: a review. Obstet Gynecol Surv. 2004. 59:368–378.
14. Reed SD, Voigt LF, Newton KM, Garcia RH, Allison HK, Epplein M, et al. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009. 113:655–662.
15. Ferenczy A, Gelfand M. The biologic significance of cytologic atypia in progestogen-treated endometrial hyperplasia. Am J Obstet Gynecol. 1989. 160:126–131.
16. Gallos ID, Shehmar M, Thangaratinam S, Papapostolou TK, Coomarasamy A, Gupta JK. Oral progestogens vs levonorgestrel-releasing intrauterine system for endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010. 203:547.e1–547.e10.
17. Haoula ZJ, Walker KF, Powell MC. Levonorgestrel intra-uterine system as a treatment option for complex endometrial hyperplasia. Eur J Obstet Gynecol Reprod Biol. 2011. 159:176–179.
18. Nilsson CG, Haukkamaa M, Vierola H, Luukkainen T. Tissue concentrations of levonorgestrel in women using a levonorgestrel-releasing IUD. Clin Endocrinol (Oxf). 1982. 17:529–536.
19. Phillips V, Graham CT, Manek S, McCluggage WG. The effects of the levonorgestrel intrauterine system (Mirena coil) on endometrial morphology. J Clin Pathol. 2003. 56:305–307.
20. Guttinger A, Critchley HO. Endometrial effects of intrauterine levonorgestrel. Contraception. 2007. 75:6 Suppl. S93–S98.
21. Horn LC, Schnurrbusch U, Bilek K, Hentschel B, Einenkel J. Risk of progression in complex and atypical endometrial hyperplasia: clinicopathologic analysis in cases with and without progestogen treatment. Int J Gynecol Cancer. 2004. 14:348–353.
22. Bese T, Vural A, Ozturk M, Dagistanli F, Demirkiran F, Tuncdemir M, et al. The effect of long-term use of progesterone therapy on proliferation and apoptosis in simple endometrial hyperplasia without atypia. Int J Gynecol Cancer. 2006. 16:809–813.
23. Varma R, Soneja H, Bhatia K, Ganesan R, Rollason T, Clark TJ, et al. The effectiveness of a levonorgestrel-releasing intrauterine system (LNG-IUS) in the treatment of endometrial hyperplasia--a long-term follow-up study. Eur J Obstet Gynecol Reprod Biol. 2008. 139:169–175.
24. Lee SY, Kim MK, Park H, Yoon BS, Seong SJ, Kang JH, et al. The effectiveness of levonorgestrel releasing intrauterine system in the treatment of endometrial hyperplasia in Korean women. J Gynecol Oncol. 2010. 21:102–105.
25. Orbo A, Arnes M, Hancke C, Vereide AB, Pettersen I, Larsen K. Treatment results of endometrial hyperplasia after prospective D-score classification: a follow-up study comparing effect of LNG-IUD and oral progestins versus observation only. Gynecol Oncol. 2008. 111:68–73.
26. Haimovich S, Checa MA, Mancebo G, Fuste P, Carreras R. Treatment of endometrial hyperplasia without atypia in peri- and postmenopausal women with a levonorgestrel intrauterine device. Menopause. 2008. 15:1002–1004.
27. Orbo A, Arnes M, Pettersen I, Larsen K, Hanssen K, Moe B. Down-regulated progesterone receptor A and B coinciding with successful treatment of endometrial hyperplasia by the levonorgestrel impregnated intrauterine system. Acta Obstet Gynecol Scand. 2010. 89:1438–1446.
28. Jensen JT, Nelson AL, Costales AC. Subject and clinician experience with the levonorgestrel-releasing intrauterine system. Contraception. 2008. 77:22–29.
29. Bachmann G, Korner P. Bleeding patterns associated with non-oral hormonal contraceptives: a review of the literature. Contraception. 2009. 79:247–258.
30. Mansour D. The benefits and risks of using a levonorgestrel-releasing intrauterine system for contraception. Contraception. 2012. 85:224–234.
31. Ceci O, Bettocchi S, Pellegrino A, Impedovo L, Di Venere R, Pansini N. Comparison of hysteroscopic and hysterectomy findings for assessing the diagnostic accuracy of office hysteroscopy. Fertil Steril. 2002. 78:628–631.
32. Gallos ID, Ofinran O, Shehmar M, Coomarasamy A, Gupta JK. Current management of endometrial hyperplasia-a survey of United Kingdom consultant gynaecologists. Eur J Obstet Gynecol Reprod Biol. 2011. 158:305–307.
33. American Association of Gynecologic Laparoscopists. AAGL practice report: practice guidelines for the diagnosis and management of endometrial polyps. J Minim Invasive Gynecol. 2012. 19:3–10.
34. Haynes PJ, Hodgson H, Anderson AB, Turnbull AC. Measurement of menstrual blood loss in patients complaining of menorrhagia. Br J Obstet Gynaecol. 1977. 84:763–768.
35. Demirkiran F, Yavuz E, Erenel H, Bese T, Arvas M, Sanioglu C. Which is the best technique for endometrial sampling? Aspiration (pipelle) versus dilatation and curettage (D&C). Arch Gynecol Obstet. 2012. 286:1277–1282.
36. Lee TS, Seong SJ, Kim JW, Ryu HS, Song ES, Nam BH. Management of endometrial hyperplasia with a levonorgestrel-releasing intrauterine system: single arm, prospective multicenter study: Korean Gynecologic Oncology Group Study (KGOG2006). Jpn J Clin Oncol. 2011. 41:817–819.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download