Abstract
Objective
To evaluate the clinical efficacy of concurrent chemoradiotherapy (CCRT) using daily low-dose cisplatin for cervical cancer.
Methods
Fifty-one patients with locally advanced cervical cancer (FIGO stage IB2, bulky IIA, IIB-IVA) who were treated with CCRT as primary therapy at Kurume University Hospital between 2000 and 2007 were retrospectively reviewed. CCRT consisted of 5 mg/m2/day of cisplatin 5 days per week, and external beam radiotherapy (EBRT) administrated to whole pelvis to 45-50.6 Gy. High-dose-rate intracavitary brachytherapy was delivered in a single dose of 4-5 Gy at point A, once a week after 20-30 Gy of EBRT.
Results
The median follow-up duration was 42 months (range, 5 to 116 months). The overall response rate was 94.1%. Five year overall survival rate was 71.5% and 46.2% in stage I or II, and stage III or IVA, respectively. During follow-up period, 30 recurrences (58.8%) were found, the local failure rate was 39%, and distant failure rate was 35.2%, and both (local and distant) were 15.7%. Hematological toxicities were the most frequent acute toxicities. Grade 3 and 4 neutropenia was observed in 37.3%. Late intestinal toxicities appeared in 7 cases (13.7%), which occurred between 6 and 114 months after treatment. Four cases required bowel surgery.
Conclusion
CCRT using daily low-dose cisplatin was tolerable and showed favorable initial response as the primary therapy for locally advanced uterine cervical cancer. But there was no remarkable long-term benefit for patients' survival or local disease control in this study. The incidence of late intestinal toxicity still requires further investigation.
Cervical cancer still shows the highest incidence and substantial mortality rate among gynecologic cancers in Japanese women. Radiation therapy is the standard treatment for locally advanced cervical cancer. In 1999, five large randomized control studies demonstrated superiority of concurrent chemoradiotherapy (CCRT) in terms of survival rate, compared with conventional radiotherapy [1-5]. After publication of these five papers, the National Cancer Institute (NCI) issued a clinical announcement stating strong consideration should be given to the incorporation of concurrent cisplatin-based chemotherapy with radiotherapy for treatment of cervical cancer. Nevertheless, the mechanism of interaction of radiotherapy and chemotherapy has not been completely elucidated, and the optimum drug dose and schedule to use remains to be discussed. We conducted CCRT with low-dose cisplatin for high-risk uterine cervical cancer to examine clinical efficacy in terms of toxicity and anti-tumor effect.
Patients with cervical cancer treated with CCRT, as primary therapy, at Kurume University Hospital between 2000 and 2007 were retrospectively reviewed. Eligibility criteria were as follows; an age less than 80, performance status 0-2, adequate renal, hepatic, and bone marrow function, International Federation of Gynecology and Obstetrics (FIGO) stage IB2, bulky IIA and IIB-IVA. Lymph node (LN) metastasis was diagnosed by computed tomography (CT) scan which was reviewed by radiologists (LN size greater than 1 cm in the short axis). Para-aortic node (PAN) positive cases were excluded in this series. Written informed consent was obtained in every patient.
All patients were treated with external beam radiotherapy (EBRT) 10 MeV X-ray by Linac and high-dose-rate intracavitary brachytherapy (HDR-ICBT). EBRT was delivered to the whole pelvic field with a dose of 1.8 Gy 5 days a week in 28 fractions totaling 50.4 Gy by anteposterior and postanterior parallel ports. HDR-ICBT was delivered in a single dose of 4-5 Gy at point A, once a week, after 20-30 Gy of EBRT with center shield. The total dose of ICBT was 15-20 Gy. Radiotherapy was stopped with the appearance of grade 4 neutropenia or grade 3 diarrhea, and resumed after recovery to grade 2.
Chemotherapy was administered on the same day just before EBRT by intravenous cisplatin of 5 mg/m2 with 500 mL sodium chloride solution in a 2 hours infusion. Chemotherapy was halted by grade 4 neutropenia or thrombocytopenia, grade 3 anemia, or other grade 3 non-hematologic toxicities. Neither prophylactic use of antiemetic drugs nor granulocyte-colony stimulating factor was administered.
The local response was evaluated by radiologic examination and pathologic examination after completion of CCRT. The overall survival rate (OS) and progression-free survival rate (PFS) were computed using the Kaplan-Meier method, and log-rank test for survival analysis. Complete response (CR) was defined as no evidence of disease by radiologic study (CT/MRI) on the completion of treatment, and pathological study (local biopsy). The hematological toxicity and non-hematological toxicities (weight loss, diarrhea, abdominal pain, nausea and vomiting) were classified by the National Cancer Institute-Common Toxicity Criteria version 3. During the follow-up period, recurrence was confirmed by radiologic study, local Pap smear or biopsy. Moreover, late intestinal toxicity was classified by Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer (RTOG/EORTC) radiation toxicity grading [6] defined as toxicity occurring at 90 days after completion of the therapy.
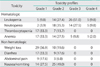
Patient characteristics are shown in Table 1. Fifty-one patients were included in this study. Fifteen patients with stage I and II were originally planned for radical hysterectomy but underwent CCRT by following reasons. Radical hysterectomy was cancelled during surgery in 5 patients because of macroscopic extrauterine disease spread. CCRT was preferred in 6 patients because of comorbidity such as cardiovascular disease, renal disease, diabetes mellitus, and high body mass index (BMI). The remaining 4 patients chose CCRT instead of radical hysterectomy by patient's will.
Most cases (88%) were squamous cell carcinoma, and 37 of 51 (72.5%) cases had bulky tumor (more than 4 cm in diameter) and median tumor size was 4.5 cm (range, 1.6 to 8 cm). Among 49 cases examined for retroperitoneal LN metastasis by imaging diagnosis or laparotomy, twenty cases were positive for pelvic LN metastasis. The median follow-up period was 42 months (range, 5 to 116 months).
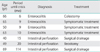
The content of treatment is shown in Table 2. The median duration of treatment was 57 days. All patients completed the scheduled dosage, but interruption of radiotherapy was recognized in three patients (5.9%) because of neutropenia, infection, and abdominal pain respectively. Chemotherapy was discontinued in four cases (7.8%), because of bone marrow suppression (3 cases), and renal dysfunction (1 case). The median dose of total administered cisplatin was 189 mg (range, 63 to 216 mg).
Table 3 summarizes the patient outcome. The overall response rate (CR+partial response [PR]) was 94.1%. Among 51 cases, 38 cases were confirmed as CR by radiologic study and no residual tumor by cervix biopsy at the completion of treatment. Stable disease (SD) was found in 3 cases (5.9%). Recurrence was found in 30 cases (30/51; 58.8%) during the follow-up period. Site of recurrences were divided into pelvic only, distant, and both pelvic and distant, and the incidences of recurrence were 23.5%, 35.2%, and 15.7%, respectively.
Stage I-II and III-IVA patients' OS and PFS are shown in Figs. 1, 2. The 5-year survival rates were 71.5% and 46.2% in stage I-II, and stage III-IVA patients, respectively, and median survival duration was 34 months for stage III-IVA patients. The rates of PFS were 58.2% and 34.6% in stage I-II and stage III-IVA, respectively. The median duration of progression-free interval was 22.0 months for all patients, and 14 months for stage III-IVA patients. In restrictive results, pelvic LN negative patients had a significantly better PFS (p=0.003) than node positive cases (Fig. 3).
There was no treatment related death in this series. Acute toxicities are shown in Table 4. Hematological toxicities were recognized most frequently. Grade 3 or 4 leucopenia was observed in 31 patients (60.8%) including grade 3 and 4 neutropenia in 14 patients (27.5%) and five (9.8%) patients, respectively. Six patients (12%) developed grade 3 or worse anemia including grade 4 in one (2%). With regard to non-hematologic toxicities, including nausea and vomiting, no other grade 3 or 4 toxicities were found.
Late intestinal toxicities developed in 7 patients (13.7%), occurring between 6 and 114 months after treatment. Four of 7 cases required bowel surgery, including three patients with life threatening intestinal perforation (Table 5).
The benefit of CCRT was shown in 16% improvement of PFS and 12% improvement of OS in cervical cancer by meta-analysis [7]. There was also a 6% improvement in 5-year survival with CCRT compared with radiotherapy in another meta-analysis [8]. Acute neutropenia and gastrointestinal toxicities were more common with CCRT but were transient and the rates of late complications were similar to radiotherapy alone [9,10]. The method of administration of cisplatin varies from study to study, and the optimum dose and method has not been established. Most studies showed survival improvement with CCRT administered with a weekly 40 to tri-weekly 75 mg/m2 dosage of cisplatin. In Gynecologic Oncology Group (GOG) Trial 123 (369 cases) with a 40 mg/m2 weekly cisplatin, grade 3 and 4 hematological toxicities were observed in 18% and 3% of patients, respectively. And in GOG Trial 120 (575 cases) with 40 mg/m2 of weekly cisplatin, 49.4% of patients were administered 6 or more cycles of cisplatin. Nevertheless, these studies did not take into consideration of ethnic difference in toxicity.
Two Japanese studies applied 40 mg/m2 of weekly cisplatin for Japanese women. Ohno et al. [11] reported on a phase 1 study of CCRT consisting of weekly cisplatin. There was no interruption of radiotherapy by cisplatin, but actual dose intensity remained at 29.3 mg/m2/week (73% of the scheduled dose) because of myelosuppression [11]. Ikushima et al. [12] reported that all patients who received 40 mg/m2 of weekly cisplatin developed grade 3 or 4 hematological toxicity and only 36% of patients completed 5 cycles of administration. On the other hand, fractionated low-dose administration of cisplatin could be adjustable, and expected to reduce toxicity. Mitsuhashi et al. [13] reported the maximum-tolerated dose of cisplatin administered daily was determined to be 8 mg/m2 in a phase I study of daily cisplatin and CCRT. Uno et al. [14] reported that 14 patients (93%) could be delivered 8 mg/m2 cisplatin daily, and the median total dose was 224 mg/m2 (range, 200 to 240 mg/m2). Thus, daily administration enables the recommended dose of cisplatin to be given (total dosage, 200 to 240 mg/m2) for Japanese women. Unlike other studies, this study had less than 200 mg of total dose of cisplatin on average, and the dose intensity of cisplatin resulted in only 25 mg/m2/week. In spite of such low-dose concentration, a favorable local response, including 94.1% of overall response rate was obtained. On the other hand, hematological toxicity of our study was relatively severe compared to the toxicities of previous western studies [15]. According to most studies, the incidences of grade 3 or 4 hematological toxicity of them were around 20%, but it was 60.8% in our study. Nevertheless, most of the non-hematologic toxicities were mild. It is unclear whether the reason of this phenomenon derived from the difference of administration or ethnic difference. Some information from the analysis of weekly CCRT study for Japanese women (JGOG 1066) is expected to answer this question.
Daily administration of cisplatin was also anticipated to work as a radiosensitizer of daily radiotherapy. Although the patients of this study included high incidence of patients with bulky tumors (73%), CCRT with low-dose daily cisplatin showed high local control rate as 94.1% as the primary treatment. However during follow-up period, we had 30 recurrences (58.8%) including 20 patients (39.2%) with local failure, which was higher than results of previously reported investigators (18-24.2%) [4,10,16,17]. Duration of radiotherapy was relatively longer in this study, but this may not be the cause of relatively higher recurrence rate of this study. A meta-analysis of 18 trials from British group could not find any survival difference according to the duration of radiotherapy [8]. This report also pointed out that the benefit of CCRT decreased with the advance of clinical stage, estimated absolute survival benefit of 10% (stage IA to IIA), 7% (stage IIB), and 3% (stage III to IVA) at 5 years [8]. Current study included larger number of stage III-IVA patients compared to the previous reports which may explain the high recurrence rate in long-term follow-up. Furthermore, there was a significant difference in the progression-free survival between patients with node negative and node positive patients. Even as a local therapy, it was obvious that this protocol could not overcome node positive disease.
On the other hand, we had 18 patients (35.2%) with distant failure. The difference of patient inclusion criteria might be a reason for high rate of distant failure. Although previous randomized controlled trial studies excluded patients with PAN metastasis by pretreatment LN dissection, such procedures were not performed on our patients. This suggests that such patients already had undetectable micro metastasis beyond the pelvis. We have to consider advanced stage or pelvic LN positive cases as systemic disease, rather than locally advanced cancer. Adjuvant chemotherapy after CCRT may improve the outcome of advanced cancer with extra pelvic disease [18,19].
Regarding late toxicity, we could not disregard our 7 cases (13.7%) of late intestinal toxicities, including 4 cases requiring surgical treatment. A follow-up report of GOG120 trial revealed no significant difference in the incidence of grade 3 or 4 gastrointestinal or urologic adverse effects between CCRT versus EBRT alone [10]. Nevertheless, Kirwan et al. [15] pointed out that many reports underestimated the incidence of late toxicity. In this study, six of seven cases of late intestinal toxicities were observed within 2 years. However, the remaining one patient experienced intestinal perforation after 9 years of treatment. So long-term and careful observation may be necessary against unknown toxicity in CCRT patients.
In conclusion, CCRT using daily low-dose cisplatin was tolerable and showed high response rate as a primary treatment for locally advanced uterine cervical cancer. However, long-term observation revealed no remarkable advantage for survival of patients and local disease control was not observed. The incidence of late intestinal toxicity still requires further investigation.
Figures and Tables
ACKNOWLEDGMENTS
We thank Dr. Kenichi Jingu of St Mary Hospital, Kurume, for brachytherapy. This study was supported by the supporting fund of Obstetrics and Gynecology, Kurume University, Japan.
References
1. Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL 3rd, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999. 340:1154–1161.
2. Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000. 18:1606–1613.
3. Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999. 340:1137–1143.
4. Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC Jr, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999. 17:1339–1348.
5. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999. 340:1144–1153.
6. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995. 31:1341–1346.
7. Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet. 2001. 358:781–786.
8. Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008. 26:5802–5812.
9. Rose PG. Chemoradiotherapy for cervical cancer. Eur J Cancer. 2002. 38:270–278.
10. Rose PG, Ali S, Watkins E, Thigpen JT, Deppe G, Clarke-Pearson DL, et al. Long-term follow-up of a randomized trial comparing concurrent single agent cisplatin, cisplatin-based combination chemotherapy, or hydroxyurea during pelvic irradiation for locally advanced cervical cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007. 25:2804–2810.
11. Ohno T, Kato S, Tsuji H. Phase I study of weekly cisplatin plus radiotherapy for locally advanced cervical cancer. J Jpn Soc Gynecol Oncol. 2005. 23:564–571.
12. Ikushima H, Osaki K, Furutani S, Yamashita K, Kawanaka T, Kishida Y, et al. Chemoradiation therapy for cervical cancer: toxicity of concurrent weekly cisplatin. Radiat Med. 2006. 24:115–121.
13. Mitsuhashi A, Uno T, Tanaka N, Suzuka K, Tate S, Yamazawa K, et al. Phase I study of daily cisplatin and concurrent radiotherapy in patients with cervical carcinoma. Gynecol Oncol. 2005. 96:194–197.
14. Uno T, Mitsuhashi A, Isobe K, Yamamoto S, Kawakami H, Ueno N, et al. Concurrent daily cisplatin and extended-field radiation therapy for carcinoma of the cervix. Int J Gynecol Cancer. 2008. 18:80–84.
15. Kirwan JM, Symonds P, Green JA, Tierney J, Collingwood M, Williams CJ. A systematic review of acute and late toxicity of concomitant chemoradiation for cervical cancer. Radiother Oncol. 2003. 68:217–226.
16. Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004. 22:872–880.
17. Choi IJ, Cha MS, Park ES, Han MS, Choi Y, Je GH, et al. The efficacy of concurrent cisplatin and 5-flurouracil chemotherapy and radiation therapy for locally advanced cancer of the uterine cervix. J Gynecol Oncol. 2008. 19:129–134.
18. Duenas-Gonzalez A, Zarba JJ, Patel F, Alcedo JC, Beslija S, Casanova L, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol. 2011. 29:1678–1685.
19. Wang S, Zhang DS, Pan T, Liu S, Wang MK. Efficacy of concurrent chemoradiotherapy plus adjuvant chemotherapy on advanced cervical cancer. Chin J Cancer. 2010. 29:959–963.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download