Abstract
Objective
To assess retrospectively the feasibility of intraoperative intraperitoneal (IP) chemotherapy with cisplatin in epithelial ovarian cancer.
Methods
IP chemotherapy during optimal staging surgery was performed in 10 patients who were diagnosed with primary epithelial ovarian cancers between April 2008 and February 2011. Cisplatin (70 mg/m2 in 1 L normal saline solution) was administered in the abdominal cavity for 24 hours postoperatively and then adjuvant chemotherapy was started 2-4 weeks after surgery. Perioperative toxicity of the combined treatment was evaluated until the initiation of postoperative adjuvant chemotherapy.
Results
A total of 23 adverse events were observed in 9 of 10 patients (grade 1, 7; grade 2, 13; grade 3, 3; grade 4, 0). In descending order of frequency, adverse events affected the gastrointestinal system (n=14), hematologic system (n=6), pulmonary system (n=2), and genito-urinary system (n=1). The adverse events did not affect adjuvant systemic chemotherapy schedules. One patient experienced disease recurrence in the liver 16 months after surgery. The remaining 9 patients have been well controlled by chemotherapy and/or observation during the follow-up period of 4 to 39 months after surgery.
Epithelial ovarian cancer is the leading cause of death from malignant gynecologic neoplasms in the developed world. Spread of the disease is most frequently through transcoelomic dissemination by exfoliation of cells along the surface of the peritoneal cavity, and most residual tumors or recurrent ovarian cancers are located in the pelvic cavity.
Until now, the standard treatment has included primary staging or cytoreductive surgery followed by systemic chemotherapy. Due to the known spread pattern of the disease, intraperitoneal (IP) chemotherapy may be considered as an additional treatment modality after cytoreductive surgery to facilitate higher tumor penetration and concentration of the cytotoxic drug in the peritoneal cavity. Based on the results of recent randomized phase III trials, a combination of intravenous (IV) and IP administration of chemotherapy shows a significant survival benefit among women with optimally debulked epithelial ovarian cancer, compared to IV administration alone [1-4].
The use of IP chemotherapy at the time of surgery and/or in the immediate postoperative period facilitates uniform drug delivery and may avoid some of the complications of prolonged peritoneal access [3]. The immediate intraoperative hyperthermic intraperitoneal chemotherapy (HIPEC) procedure has the potential to maximize the strengths and minimize the weaknesses associated with IP chemotherapy, including catheter-related complications. The addition of heat augments the cytotoxic effect of many chemotherapeutic agents through pharmacokinetic mechanisms such as increased cell membrane permeability, improved membrane transport, destructive capacity by elevated lysosomal enzyme activity in malignant cells, and temperature-dependent increases in drug action [5,6]. A systematic review of the available data of phase II trials of HIPEC suggested promising overall survival outcomes (range, 22 to 54 months) [3]. However, HIPEC requires additional equipment such as heater, pumps, etc., lengthens the operation time by at least 1-2 hours, and is highly expensive. Therefore, we introduced intraoperative IP chemotherapy immediately after completion of the cytoreductive surgery in treating epithelial ovarian cancer. This pilot study was performed to assess the feasibility of intraoperative IP chemotherapy with cisplatin in epithelial ovarian cancer.
IP chemotherapy during surgery in patients who were diagnosed with primary epithelial ovarian cancers and underwent an optimal staging procedure was performed at CHA Gangnam Medical Center, CHA University between April 2008 and February 2011. Eligibility criteria for inclusion in the study were as follows: patients diagnosed with primary epithelial ovarian cancer; FIGO stages IC to IV; Eastern Cooperative Oncology Group (ECOG) performance status 0 to 1; underwent comprehensive staging procedures; and achieved optimal cytoreductive surgery.
All patients had a preoperative physical examination, transvaginal ultrasonography and an abdomino-pelvic CT. Patients with good performance status were considered for surgery. Perioperative antibiotics and postoperative thromboembolic prophylaxis with low molecular-weight heparin and pressure stockings were routinely used.
Optimal cytoreductive surgery was defined as the absence of gross tumor lesion after the procedures. To eradicate all macroscopic tumors, standard staging procedures including hysterectomy, salpingo-oophorectomy, omentectomy and lymph node dissection or debulking surgery such as mass excision were performed. The largest dimension of the largest residual tumor after surgery was agreed on among the attending surgeons.
IP chemotherapy was administered through open or laparoscopic procedures according to the surgical approach. A cisplatin dose of 70 mg/m2 in 1 L normal saline solution was administered into the abdominal cavity. Two closed drains (Barovac; Sewoon Medical Co., Cheonan, Korea) remained in the pelvic cavity. Twenty-four hours after the operation, intraabdominal drain locks were released to remove the cytotoxic agent.
Prophylactic antibiotics were administered intravenously and intramuscularly for 7 days postoperatively (third generation cephalosporin, aminoglycoside, metronidazole) followed by prescription of oral antibiotics. Intravenous Patient-Controlled Analgesia (drug mixture of Fentanyl 400 µg and Ketorolac tromethamine 120 mg in 5% dextrous water 78 mL, continuously infused at 2 mL/hour via infusion pump [WalkMed Infusion, Englewood, CO, USA] for 2 days) was used to relieve postoperative pain. Patients were allowed sips of water after passing gas and a clear liquid diet was offered as the first meal on postoperative day 3. The next meal was a soft diet and then patients were offered a general diet.
The perioperative toxicity was evaluated during the period from the end of this combined treatment to the initiation of postoperative adjuvant chemotherapy. The database included adverse events assigned by organ system as described by Yan et al. [7], Washington Cancer Institute. For each adverse event, a grade 1 to 4 score with increasing severity was assigned as follows: a grade 1 adverse event required no treatment; a grade 2 adverse event required medical treatment; a grade 3 adverse event was potentially serious, but resolved conservatively with invasive intervention, such as a CT or ultrasound-guided diagnostic or therapeutic procedure; and a grade 4 adverse event required a definitive urgent intervention such as reoperation or return to the intensive care unit. All data were collected retrospectively through chart review. The Institutional Review Board of CHA Gangnam Medical Center approved this study.
All statistical calculation and analysis was done by using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA).
This study included 10 consecutive patients with primary ovarian cancer who underwent IP chemotherapy immediately after optimal cytoreductive surgery. All procedures were performed by the same surgeon (SJS). Table 1 demonstrates the patient characteristics. Median age was 41 years (range, 28 to 52 years), and median body mass index was 21.6 (range, 17.3 to 27.3). One patient (10%) had coexisting hypertension that was well controlled and did not pose a marked impairment for the combined treatment. No patient received neoadjuvant chemotherapy. The baseline median CA-125 level was 51.4 U/mL (range, 10.0 to 12,860.0 U/mL). The stages of ovarian carcinoma were IC (n=6), IIIB (n=1), and IIIC (n=3). The histology of ovarian carcinoma was as follows: serous carcinoma (n=4), mucinous carcinoma (n=2), endometrioid carcinoma (n=2), and clear cell carcinoma (n=2).
Table 2 shows the operative procedures for ovarian cancer. All 10 patients underwent salpingo-oophorectomy, pelvic lymph node dissection, and omentectomy; 6 patients had surgery by laparotomy and 4 patients by laparoscopy.
Total abdominal or laparoscopic hysterectomies were performed in 7 patients (abdominal 6 and laparoscopic 1, respectively). Three patients who had no children did not undergo hysterectomy to preserve fertility. Five patients, including the 3 who did not have hysterectomies, underwent unilateral salpingo-oophorectomy. One patient among these 5 patients underwent unilateral salpingo-oophorectomy because she had undergone contralateral adnexectomy previously. The other 5 patients underwent bilateral salpingo-oophorectomy during the study period.
Nine patients underwent paraaortic lymph node dissection. Eight patients underwent appendectomy while 1 patient had undergone appendectomy previously. One patient underwent resection of bladder peritoneum and rectal serosa for suspicious small cancerous masses in those areas.
The median duration of surgical procedures with IP chemotherapy was 340 minutes (range, 210 to 420 minutes). The median blood loss was 500 mL (range, 200 to 2,000 mL) (Table 3). The blood loss of 2 patients exceeded 1,000 mL and 6 patients, including these, were given transfusion intraoperatively and/or postoperatively. The median interval to passage of flatus was 2 days (range, 1 to 3 days). A Foley catheter was removed 3 days after surgery (range, 2 to 5 days). The median length of hospital stay was 16 days (range, 6 to 23 days).
Postoperative chemotherapy was started in days 12-26 postoperatively. Nine patients received intravenous chemotherapy: 8 with paclitaxel (175 mg/m2) and carboplatin (AUC 5) and 1 with cisplatin (50 mg/m2), doxorubicin (50 mg), and cyclophosphamide (200 mg). Only 1 patient who was diagnosed as stage IC and had no evidence of residual disease refused additional systemic chemotherapy and was followed up closely without treatment.
Among patients who received postoperative chemotherapy, 5 received the planned 6 cycles of paclitaxel and carboplatin regimen. One patient with a stage III tumor received 5 cycles of belotecan (a camptothecin analogue) as second-line chemotherapy because of the disease progression after 6 cycles of paclitaxel and carboplatin. This patient received another 6 cycles of docetaxel and cisplatin as third-line treatment due to liver metastasis which occurred 16 months after surgery. One patient received only 3 cycles of paclitaxel and carboplatin because image study and CA-125 were continuously within normal ranges. One patient only recently finished 3 cycles of adjuvant chemotherapy and is scheduled to complete 6 cycles.
One patient with cisplatin, doxorubicin, and cyclophosphamide chemotherapy has finished 6 cycles and the image study and CA-125 were continuously within normal ranges.
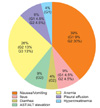
Table 4 demonstrates the incidence of perioperative adverse events by 4 organ systems. A total of 23 adverse events of grade 1 to 3 were observed in 9 of 10 patients. In descending order of frequency, adverse events affected the gastrointestinal system (14 events in 9 patients), hematologic system (6 events in 6 patients), pulmonary system (2 events in 2 patients), and genito-urinary system (1 event). Mortality was not observed in the study. Fig. 1 shows the incidence of each adverse event.
The median number of adverse events was 2 (range, 0 to 5) in patients who had IP chemotherapy after surgery. Four patients experienced 7 grade 1 adverse events that were self-limiting. In descending order of frequency, these grade 1 adverse events included transient nausea or vomiting, which was controlled by discontinuation of patient-controlled anesthesia (n=2); ileus with minimal symptoms controlled by taking nothing by mouth and hydration (n=2); high serum creatinine (n=1); aspartate aminotransferase/alanine aminotransferase (AST/ALT) elevation without clinical significance and normalized without medication (n=1); and asymptomatic pleural effusion (n=1). Nine patients experienced 13 grade 2 adverse events, which required medical treatment for resolution. In descending order of frequency, these grade 2 adverse events included nausea or vomiting requiring antiemetics (n=7); anemia requiring transfusion of ≤4 U (n=3); tolerable diarrhea, but ≥2 days' duration (n=1); AST/ALT elevation requiring hepatotonics (n=1); and pleural effusion requiring diuretics (n=1). Three patients experienced 3 grade 3 adverse events which required invasive medical intervention for resolution. In this study, grade 3 adverse events included only anemia requiring transfusion of >4 U (n=3). There was no grade 4 adverse event.
The objective of this pilot study was to investigate the feasibility of intraoperative IP chemotherapy immediately after optimal cytoreductive surgery in primary epithelial ovarian cancer patients, with a focus on associated postoperative morbidity and toxicity. The majority of events (87%, 20 of 23 events; 9 of 10 patients) were grades 1 or 2, which were self-limiting or resolved with medical treatment. Grade 3 events (13%, 3 of 23 events; 3 of 10 patients) were all anemia, which was well controlled by transfusion. A total of 6 patients (grade 2, n=3 and grade 3, n=3) were anemic perioperatively. The anemia was due to surgical complications, rather than IP chemotherapy, as 4 patients were given transfusion intraoperatively and 2 developed anemia between postoperative day 1 and 4. Most hematologic complication after chemotherapy occurs 7-10 days later and there was no leucopenia or thrombocytopenia observed in this study. No patient required reoperation or admission to the intensive care unit, and no mortality was observed in the present study.
Recently, several studies including Armstrong et al. [4], Markman et al. [8], and the National Cancer Institute (USA) [9], announced the survival benefit of IP chemotherapy; however, this procedure has 3 main complications: problems related to the access device, abdominal pain with infusion, and intolerance to the higher doses of cisplatin [10,11]. In the GOG 172 study, the authors observed catheter-related complications in 39 of 119 patients (33%) [12], of which 21 were due to catheter infection. Our study did not use infusing catheters; therefore, there were no complications related to the access device. In conventional IP chemotherapy, the cytotoxic drug is infused in 2 L of fluid to reach tumor lesion easily in abdominal cavity; therefore, patients experience gastrointestinal complications such as abdominal discomfort or pain, nausea, vomiting, bowel perforation, infection, or ileus. Armstrong et al. [4] reported gastrointestinal events in 46% of patients and Yan et al. [7] reported nausea/vomiting (grade 2, n=4, grade 3, n=3 in 23 patients) and intrabdominal infection (grade 3, 10%, grade 4, 24%). In our study, there was no severe abdominal pain and all gastrointestinal toxicities were grade 1 or 2. This may be because we infused cisplatin with a smaller volume of normal saline (1 L) and contained the chemoagent for a relatively short time (24 hours) in the abdominal cavity.
The morbidity of this study was no more serious than HIPEC with cytoreductive surgery studies. In a review article of 15 studies, the morbidity was 21.3-60%, including significant complication, while mortality was up to 10% [6]. Another review article analyzed morbidity of 45-60%, including minor complications, and a mortality of 3.3-10% [3]. The studies show significant morbidity such as complications requiring return to the surgical intensive care unit, an invasive procedure, or return to the operating room. Such events and mortality were not observed in this study. In a recent prospective phase II study, Lim et al. [13] reported 107 adverse events in 30 patients (grade I, 40 events; grade 2, 46 events; grade 3, 21 events). Grade 3 events included anemia (5 of 21 grade 3 events), pleural effusion (4 of 21), wound infection (3 of 21), pneumothorax (3 of 21), etc. Though the IP chemotherapy immediately after cytoreductive surgery designed in our study could not provide patients with the benefit of the heating effect on the chemotherapeutics like HIPEC, this procedure is simple, easily reproducible without any additional equipment and operation time, inexpensive, and there is less exposure of the medical team to cytotoxic chemoagents.
In a review article of 16 studies on HIPEC with cytoreductive surgery in ovarian cancer, Dovern et al. [14] reported that survival data ranged from 10.0 to 57.1 months (disease-free survival) and from 19.0 to 76.1 months (overall survival). In addition, Chua et al. [15] reported that overall median survival ranged from 22 to 64 months, with median disease-free survival ranging from 10 to 57 months. The present study was performed from April 2008 and the survival data is not yet complete. No mortality was observed during the follow-up period. One patient had liver metastasis 16 months postoperatively. The other 9 patients have been well controlled by chemotherapy and observed with a median survival of 16 months (range, 4 to 39 months) and the progression free survival of 13 months (range, 4 to 22 months).
Our institution is a secondary care hospital specializing in obstetrics and gynecology. For this reason, the present study has several limitations. First, the proportion of early stage disease is relatively high; 6 of the 10 enrolled patients were stage IC. This affects intraoperative or postoperative complication rates. In conventional IP chemotherapy and HIPEC trials mainly targeted for patients of epithelial ovarian cancer with advanced stage, bowel surgery is often required (IP 30% in Walker et al. [12]; HIPEC 83% in Lim et al. [13]). As a result such studies reported more severe morbidities than this study in which no colon surgery was required. Second, the sample size is small. However, this simple intraoperative chemotherapy can be adopted in secondary care hospitals, which have no additional equipment for HIPEC or monitoring team for conventional postoperative IP chemotherapy.
In conclusion, intraoperative IP chemotherapy with cisplatin during surgical procedures is feasible and relatively safe for the treatment of primary epithelial ovarian cancer. Morbidity and toxicity of the regimen of this study design were acceptable. Further studies, including long term prospective and comparative trials, are needed to validate the efficacy of intraoperative IP chemotherapy in epithelial ovarian cancer.
Figures and Tables
Fig. 1
Incidence (%) of 23 grade (G) 1 to 3 adverse events in patients with primary epithelial ovarian cancer who underwent cytoreductive surgery followed by intraoperative intraperitoneal (IP) chemotherapy. Nine of 10 patients experienced more than one adverse event of which 87% were grade 1 or 2. AST/ALT, aspartate aminotransferase/alanine aminotransferase.
References
1. Gadducci A, Carnino F, Chiara S, Brunetti I, Tanganelli L, Romanini A, et al. Intraperitoneal versus intravenous cisplatin in combination with intravenous cyclophosphamide and epidoxorubicin in optimally cytoreduced advanced epithelial ovarian cancer: a randomized trial of the Gruppo Oncologico Nord-Ovest. Gynecol Oncol. 2000. 76:157–162.
2. Yen MS, Juang CM, Lai CR, Chao GC, Ng HT, Yuan CC. Intraperitoneal cisplatin-based chemotherapy vs. intravenous cisplatin-based chemotherapy for stage III optimally cytoreduced epithelial ovarian cancer. Int J Gynaecol Obstet. 2001. 72:55–60.
3. Bijelic L, Jonson A, Sugarbaker PH. Systematic review of cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis in primary and recurrent ovarian cancer. Ann Oncol. 2007. 18:1943–1950.
4. Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006. 354:34–43.
5. Gonzalez-Moreno S, Gonzalez-Bayon LA, Ortega-Perez G. Hyperthermic intraperitoneal chemotherapy: rationale and technique. World J Gastrointest Oncol. 2010. 2:68–75.
6. Frenel JS, Leux C, Pouplin L, Ferron G, Berton-Rigaud D, Bourbouloux E, et al. Oxaliplatin-based hyperthermic intraperitoneal chemotherapy in primary or recurrent epithelial ovarian cancer: a pilot study of 31 patients. J Surg Oncol. 2011. 103:10–16.
7. Yan TD, Zappa L, Edwards G, Alderman R, Marquardt CE, Sugarbaker PH. Perioperative outcomes of cytoreductive surgery and perioperative intraperitoneal chemotherapy for non-appendiceal peritoneal carcinomatosis from a prospective database. J Surg Oncol. 2007. 96:102–112.
8. Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001. 19:1001–1007.
9. NCI clinical announcement: intraperitoneal chemotherapy for ovarian cancer [Internet]. National Cancer Institute. 2006. cited 2012 Mar 1. Bethesda, MD: National Cancer Institute;Available from: http://ctep.cancer.gov/highlights/docs/clin_annc_010506.pdf.
10. Markman M, Walker JL. Intraperitoneal chemotherapy of ovarian cancer: a review, with a focus on practical aspects of treatment. J Clin Oncol. 2006. 24:988–994.
11. Kim SW, Kim YT, Kim JW. New paradigm of intraperitoneal chemotherapy in ovarian carcinoma. Korean J Gynecol Oncol. 2006. 17:1–14.
12. Walker JL, Armstrong DK, Huang HQ, Fowler J, Webster K, Burger RA, et al. Intraperitoneal catheter outcomes in a phase III trial of intravenous versus intraperitoneal chemotherapy in optimal stage III ovarian and primary peritoneal cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2006. 100:27–32.
13. Lim MC, Kang S, Choi J, Song YJ, Park S, Seo SS, et al. Hyperthermic intraperitoneal chemotherapy after extensive cytoreductive surgery in patients with primary advanced epithelial ovarian cancer: interim analysis of a phase II study. Ann Surg Oncol. 2009. 16:993–1000.
14. Dovern E, de Hingh IH, Verwaal VJ, van Driel WJ, Nienhuijs SW. Hyperthermic intraperitoneal chemotherapy added to the treatment of ovarian cancer: a review of achieved results and complications. Eur J Gynaecol Oncol. 2010. 31:256–261.
15. Chua TC, Robertson G, Liauw W, Farrell R, Yan TD, Morris DL. Intraoperative hyperthermic intraperitoneal chemotherapy after cytoreductive surgery in ovarian cancer peritoneal carcinomatosis: systematic review of current results. J Cancer Res Clin Oncol. 2009. 135:1637–1645.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download