Abstract
A 48-year-old woman underwent total abdominal hysterectomy with conservation of the ovaries and tubes. Histology showed a well-circumscribed smooth muscle tumor with foci of degeneration (including infarct-type necrosis) but no coagulative tumor cell necrosis and only mild focal cytological atypia. She presented, 24 years later with shortness of breath and abdominal distension and underwent bilateral salpingo-oophorectomy, appendectomy, omental biopsy and para-aortic lymph node sampling. Histology showed bilateral ovarian smooth muscle tumors with no coagulative tumor cell necrosis or significant cellular atypia. The cells were mitotically active. The tumors in both ovaries were most likely secondary to the previous uterine smooth muscle neoplasm. To our knowledge, this case is the first in the literature to describe a benign cellular leiomyoma that subsequently behaved as a smooth muscle tumor of uncertain malignant potential, which recurred 24 years after the initial diagnosis.
Smooth muscle tumors of uncertain malignant potential (STUMP) are a heterogeneous group of neoplasms, from both the histological and clinical point of view. The clinical behaviour of these neoplasms is also poorly understood. The majority of cases follow a benign clinical course, however a few can metastasize as either tumor of low malignant potential or leiomyosarcomas. Occasionally, certain types of benign leiomyoma can follow a clinical course that supports their malignant potential. We describe a case of a benign cellular leiomyoma that subsequently behaved as a STUMP.
A 48-year-old woman was referred to the gynecological clinic following the diagnosis of a pelvic mass during an opportunistic health check. She was asymptomatic and otherwise fit and healthy. In her family history her mother had been diagnosed with breast cancer at the age of 46 years.
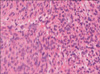
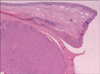
Examination revealed an enlarged fibroid uterus to the size of a 16-week pregnancy. She underwent total abdominal hysterectomy with conservation of the ovaries and tubes. Histology showed a well-circumscribed smooth muscle tumor (cellular variant) with foci of degeneration (including infarct-type necrosis) but no coagulative tumor cell necrosis and only mild focal cytological atypia (Fig. 1). The mitotic rate averaged 2 per 10 high power fields (HPF, Nikon 80i, ×400 magnification).
She presented, 24 years later with shortness of breath and abdominal distension. A CT scan of the abdomen and pelvis showed large, bilateral complex pelvic masses and a moderate amount of ascites. No other abnormality was detected. A CT pulmonary angiogram showed bilateral pulmonary emboli. Serum CA-125 level was 1,250 kU/L (normal range, 0 to 35 kU/L).
She underwent laparotomy where a large volume of ascites was drained. A 20 cm, mobile right ovarian mass and a 15 cm left ovarian mass attached to pelvic side wall were noted. No abnormalities were detected on the peritoneal surfaces, liver, spleen and diaphragm. Bilateral salpingo-oophorectomy, appendectomy, omental biopsy and para-aortic lymph node sampling was performed. Postoperative recovery was uncomplicated.
Histology showed bilateral ovarian smooth muscle tumors. There was no coagulative tumor cell necrosis or significant cellular atypia (Fig. 2). The cells were mitotically active, averaging 6 mitoses per 10 HPF, but up to 12 per 10 HPF in the more cellular active areas (Nikon 80i, ×400 magnification). Immunohistochemically, the cells showed strong positivity for desmin with focal staining for CD 10. There was no staining for inhibin, CD 34, CD 117 or calretinin. The appendix, omentum, para-aortic lymph nodes and peritoneal washings showed no abnormalities.
Smooth muscle tumors of uncertain malignant potential are a heterogeneous group of neoplasms, from both the histological and clinical point of view. Due to the rarity of these tumors, the literature on the topic is limited; a consensus on their diagnosis, malignant potential, monitoring and treatment has still not been reached [1-5].
The diagnosis of these tumors is often challenging, as interpretative difficulties and subjectivity can be encountered when analysing any of the three histological features: cellular atypia, mitotic index and coagulative tumor cell necrosis. These three features are called the Stanford criteria and were developed by Bell et al. [2].
The clinical behaviour of these neoplasms is also poorly understood. The majority of cases follow a benign clinical course, however a few can metastasize as either tumor of low malignant potential or leiomyosarcomas. This seems to happen regardless of the initial surgical procedure and in different locations. Recurrences behave in a low-grade malignant fashion, following a disease-free interval and with a prolonged survival, even when they recur as leiomyosarcomas.
In our case, the neoplasms within both ovaries were morphologically similar. They had a nodular architecture and were composed of bland spindle shaped cells. Scattered mitotic figures were present but these were sparse. There were areas of edema and hemorrhage but no evidence of coagulative tumor cell necrosis. Hemosiderin laden macrophages were present focally secondary to hemorrhagic degeneration. The cells showed diffuse staining with desmin and H-caldesmon consistent with smooth muscle tumors, and were negative for CKIT and DOG1, excluding a gastrointestinal stromal tumor.
The tumors in both ovaries were most likely secondary to the previous uterine smooth muscle neoplasm. Although a separate primary within both ovaries was a possibility, it is highly unusual for primary ovarian smooth muscle neoplasms to be bilateral. Although there was no definite coagulative tumor cell necrosis in the initial tumor, given the subsequent course of events the tumor has behaved as very low grade malignancy, even though it did not fit the Stanford criteria.
Very few studies have analysed STUMPs with recurrences. Different histological classifications (not always using the Stanford criteria), diagnostic methods, length of follow-up and lack of detailed histological information make it difficult to compare the findings and draw conclusions. Even though all the reported cases of recurrent STUMPs survived (follow-up ranging from post-operative status to 157 months following the initial diagnosis), most of the results from the literature are controversial. There seems to be no consensus as to which histological features of STUMPs predict a higher probability of recurrence, the location of recurrence (sites reported include pelvis, abdomen, liver, lungs, lymph nodes, humerus, retroperitoneum and uterus-if hysterectomy not performed), time to recurrence (anywhere between 15 months to 9 years) and histological type of recurrences (STUMP or leiomyosarcoma) [2,4,6-9]. No demographic characteristics (age, ethnicity, tobacco use) are predictive of recurrences [4]. A few studies have identified immunohistochemical markers as a predictor of recurrences. Poorer prognosis is associated with the presence of p16 and p53 immunohistochemical positivity [1,10].
To our knowledge, this case is the first in the literature to describe a benign cellular leiomyoma that subsequently behaved as a STUMP, which recurred 24 years after the initial diagnosis. As the primary tumor occurred before the publication of the Stanford criteria, the diagnosis of necrotic smooth muscle neoplasm of low malignant potential was made. According to Bell and colleagues [2], the initial tumor should have been classified as a "benign leiomyoma". Even though the histological features do not fit the diagnosis of STUMP, we believe that the clinical behaviour of this tumor did, and the original diagnosis was both prescient and correct. Its ability to metastasize 24 years later and the clinical presentation with bilateral pulmonary emboli, all support the malignant potential of this neoplasm. The pulmonary emboli could have been the result of either malignancy-induced hypercoagulability or intravascular lung metastases; it was not possible to differentiate between the two on imaging.
In conclusion, the classification of STUMPs is still controversial and perhaps even some types of "benign leiomyomas" should be added to the list. Being able to find markers to predict the malignant potential of these neoplasms will be highly beneficial, in order to reach a consensus on their diagnosis and be able to manage them appropriately. Further studies are required to increase the validity of the current literature and add more knowledge on the subject. Future hope lies in the identification of immunohistochemical, molecular and genetic markers for the prediction of recurrent potential of STUMPs.
Figures and Tables
References
1. Atkins KA, Arronte N, Darus CJ, Rice LW. The Use of p16 in enhancing the histologic classification of uterine smooth muscle tumors. Am J Surg Pathol. 2008. 32:98–102.
2. Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms: a clinicopathologic study of 213 cases. Am J Surg Pathol. 1994. 18:535–558.
3. Berretta R, Rolla M, Merisio C, Giordano G, Nardelli GB. Uterine smooth muscle tumor of uncertain malignant potential: a three-case report. Int J Gynecol Cancer. 2008. 18:1121–1126.
4. Guntupalli SR, Ramirez PT, Anderson ML, Milam MR, Bodurka DC, Malpica A. Uterine smooth muscle tumor of uncertain malignant potential: a retrospective analysis. Gynecol Oncol. 2009. 113:324–326.
5. D'Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010. 116:131–139.
6. Shapiro A, Ferenczy A, Turcotte R, Bruchim I, Gotlieb WH. Uterine smooth-muscle tumor of uncertain malignant potential metastasizing to the humerus as a high-grade leiomyosarcoma. Gynecol Oncol. 2004. 94:818–820.
7. Amant F, Moerman P, Vergote I. Report of an unusual problematic uterine smooth muscle neoplasm, emphasizing the prognostic importance of coagulative tumor cell necrosis. Int J Gynecol Cancer. 2005. 15:1210–1212.
8. Ip PP, Tse KY, Tam KF. Uterine smooth muscle tumors other than the ordinary leiomyomas and leiomyosarcomas: a review of selected variants with emphasis on recent advances and unusual morphology that may cause concern for malignancy. Adv Anat Pathol. 2010. 17:91–112.
9. Ng JS, Han A, Chew SH, Low J. A clinicopathologic study of uterine smooth muscle tumors of uncertain malignant potential (STUMP). Ann Acad Med Singapore. 2010. 39:625–628.
10. Ip PP, Cheung AN, Clement PB. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): a clinicopathologic analysis of 16 cases. Am J Surg Pathol. 2009. 33:992–1005.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download