Abstract
Cancers of an unknown primary site are heterogenous with respect to their clinical and pathologic features. They are generally very aggressive, but specific favorable subsets have a better prognosis. For these favorable subsets, taxane based chemotherapy is very effective for a subset of woman with papillary serous peritoneal adenocarcinoma. A 52 year-old woman underwent [18F]-FDG PET/CT for routine health screening. On PET/CT, multiple hypermetabolic lymph nodes were detected in the paraaortic spaces, and there were no other hypermetabolic abnormalities. The patient was diagnosed with an unknown primary cancer that probably originated from the ovary or peritoneum, according to clinical studies and biopsy results. This was not a typical case of a favorable subset of cancer of an unknown primary site, but the tumor showed complete biologic response to taxane based chemotherapy as revealed by PET/CT, and necrotic tumor cells were confirmed by surgery.
Cancer of an unknown primary site (CUPS) is defined as a biopsy proven metastatic malignant tumor whose primary site cannot be identified during the pretreatment evaluation. Early dissemination, the unpredictability of the metastatic pattern, and aggressiveness constitute the fundamental characteristics of these tumors [1,2]. CUPS have various heterogenous clinical and pathologic features, yet within this diverse group there are specific clinical and/or pathologic features that can be used to define several subsets of patients who have favorable prognoses [3]. For these favorable subsets, taxane based chemotherapy was very effective for a subset of woman with papillary serous peritoneal adenocarcinoma. We describe here an unusual case of CUPS that manifested with large multiple intraabdominal lymph nodes. We successfully treated the patient with taxane based chemotherapy and surgery.
A 52 year old woman underwent [18F]-FDG PET/CT for routine health screening. She had suffered from vague abdominal discomfort. She had no significant past medical history. On PET/CT, multiple hypermetabolic lymph nodes encasing the inferior vena cava and abdominal aorta were detected (SUVmax, 14.9 in the retrocaval area), and there were no other hypermetabolic abnormalities (Fig. 1A). On physical examination, there was mild tenderness on the mid-abdomen. The laboratory examinations were within normal ranges including complete blood count, renal function tests, and liver function tests. Of the tumor marker tests, only CA-125 was elevated to 250 U/mL. Neck, chest, and abdominal CT, pelvic MRI, gastroduodenoscopy and colonoscopy were performed for further evaluation. The gastroduodenoscopy and colonoscopy were negative. Abdominal CT showed about 6 cm sized multiple lymph nodes encasing the abdominal aorta (Fig. 2A). There were no abnormalities in both ovaries by pelvic MRI. She underwent a laparoscopic surgical biopsy. The results showed metastatic carcinoma and the tumor cells were positive for cytokeratin (CK), CK7, estrogen receptor (ER), CA-125 and Wilm's tumor-1 (WT-1) on immunohistochemical staining (Fig. 3A, B). The other immunohistochemical stains were negative, including gross cystic disease fluid protein-15 (GCDFP-15), CK20 and progesterone receptor (PR). The patient was diagnosed with cancer of an unknown primary site, and it probably originated from the ovary or peritoneum on the basis of the clinical studies and biopsy results. We thought that the tumor mass could not be completely resected due to encasement of the great vessels, and therefore combination chemotherapy that consisted of paclitaxel and carboplatin was started. The follow-up CT after 6 cycles of chemotherapy showed marked partial response according to the RECIST criteria, that is, the tumor decreased from 6×5 cm to 2×2 cm, and so we planned a debulking operation (Fig. 2B). Preoperative PET/CT showed no hypermetabolism in the remnant lesion (Fig. 1B). The patient underwent transabdominal hysterectomy, both salpingo-oophorectomy and pelvic/paraaortic lymph nodes dissection. The operative biopsy results showed only necrotic cell debris in the lymph nodes without tumor cells, and there were no other tumor cells in the female genital organs (Fig. 3C). An additional 6 cycles of chemotherapy were administered after surgery. She is on follow-up without evidence of recurrence.
For CUPS, the specific favorable subsets are treatable, and initial identification these patients is imperative for their optimal management. At least five of these favorable subsets have clinical features similar to specific cancer types: 1) women with axillary lymph node metastasis, breast cancer; 2) men with blastic bone metastasis and tumor staining or elevated serum levels of prostate specific antigen, prostate cancer; 3) young men with the extragonadal germ cell syndrome, germ cell tumor; 4) isolated neck nodes involved with squamous cell carcinoma, head and neck primary cancer; 5) woman with papillary serous peritoneal adenocarcinoma, primary peritoneal carcinoma or ovary cancer. Therapy directed at the presumed primary benefits these patients, and this supports the idea that at least some CUPS retain similar sensitivity to therapies that are known to be useful for the known primaries [2]. A woman with papillary serous peritoneal adenocarcinoma has clinical features similar to those for advanced ovarian cancer including ascites, tumor involvement usually limited to the peritoneal surfaces and elevated serum levels of CA-125, yet there is no evidence of primary tumor in the ovaries [4]. These patients should be managed as stage III ovarian cancer with taxane/platinum based systemic chemotherapy. The median complete response rate is 20%, the median survival is 16 months and the median long term survival (>2 years) is 16% [3,5].
This was not a typical case of a favorable subset of CUPS. This patient manifested with only huge multiple intraabdominal lymph nodes, yet we chose taxane based chemotherapy according to the immunohistochemical results. The main positive markers for ovarian adenocarcinoma are ER, CA-125, mesothelin, and WT-1, and the diagnosis of primary ovarian tumors is favored by the CK7+/CD20- [6]. The immunohistochemical results of the tumor cells in this case were CK7+/CK20-, ER+, CA-125+, and WT-1+. Besides, the tumors showed complete biologic response to taxane based chemotherapy as revealed by PET/CT and necrotic tumor cells were confirmed by surgery.
Imaging has a fundamental role in oncology, especially for assessing the tumor response to therapy. The current conventional imaging follow-up is based on morphological criteria with the changes of the tumor dimensions determining a response or tumor progression. PET/CT provides an indication of the metabolic and proliferative activity within tumors. It is useful in the early evaluation of a tumor response because changes in the gross tumor size are notably delayed, and they substantially lag behind the biological and molecular changes that are known to occur early in responders [7,8]. Monitoring the early treatment response will allow modifying and adapting treatment on the basis of the patient's treatment response. Ultimately, the aim is to improve the outcomes and reduce the acute and late treatment-related side effects to achieve the best possible therapeutic gain and quality of life. PET/CT has been recently widely used to evaluate the tumor response to chemotherapy for various cancers such as esophageal cancer, head and neck cancer, lung cancer, breast cancer and lymphoma. It can also be useful for an unknown primary cancer. In this case, we performed PET/CT for evaluating the extent of disease and the treatment response to chemotherapy before the debulking operation.
In conclusion, immunohistochemical evaluation of CUPS to predict the primary sites is very important for the optimal treatment, and PET/CT can also be used for CUPS to evaluate the tumor response to chemotherapy.
Figures and Tables
Fig. 1
(A) The [18F]FDG PET/CT showed multiple conglomerated hypermetabolic lymph nodes encasing the inferior vena cava and abdominal aorta in the retrocaval, portocaval and paraaortic spaces. (B) The preoperative [18F]FDG PET/CT revealed that there were no hypermetabolic lesions in the remnant lymph nodes.
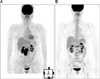
Fig. 2
(A) The initial CT showed huge intraabdominal lymph nodes encasing the inferior vena cava and abdominal aorta. (B) The follow-up CT after 6 cycles of taxane based chemotherapy showed a marked partial response according to the RECIST criteria.
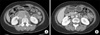
Fig. 3
(A) The laparoscopic biopsy results showed the tumor cells that have pleomorphic nuclei and small cytoplasm (H&E, ×100). (B) The immunohistochemical staining results showed the tumor cells were positive for Wilm's tumor-1. (C) The operative biopsy results showed only extensive necrosis in the remnant lymph nodes without tumor cells (H&E, ×10).

References
1. Pavlidis N. Cancer of unknown primary: biological and clinical characteristics. Ann Oncol. 2003. 14:Suppl 3. iii11–iii18.
2. Greco FA, Hainsworth JD. Introduction: unknown primary cancer. Semin Oncol. 2009. 36:6–7.
3. Hainsworth JD, Fizazi K. Treatment for patients with unknown primary cancer and favorable prognostic factors. Semin Oncol. 2009. 36:44–51.
4. Fromm GL, Gershenson DM, Silva EG. Papillary serous carcinoma of the peritoneum. Obstet Gynecol. 1990. 75:89–95.
5. Dalrymple JC, Bannatyne P, Russell P, Solomon HJ, Tattersall MH, Atkinson K, et al. Extraovarian peritoneal serous papillary carcinoma: a clinicopathologic study of 31 cases. Cancer. 1989. 64:110–115.
6. Dennis JL, Hvidsten TR, Wit EC, Komorowski J, Bell AK, Downie I, et al. Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin Cancer Res. 2005. 11:3766–3772.
7. Harry VN, Semple SI, Parkin DE, Gilbert FJ. Use of new imaging techniques to predict tumour response to therapy. Lancet Oncol. 2010. 11:92–102.
8. Pickles MD, Gibbs P, Lowry M, Turnbull LW. Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn Reson Imaging. 2006. 24:843–847.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download