Abstract
Sweet's syndrome, or acute febrile neutrophilic dermatosis, is a condition characterized by fever, neutrophilia, erythematous skin lesions, and a dermal infiltrate consisting predominantly of mature neutrophils on histology. Sweet's syndrome is a reactive phenomenon and should be considered a cutaneous marker of systemic disease, including underlying malignancy. We present a case of a 56-year-old woman who presented with vague abdominal symptoms and a tender, erythematous rash on her extremities. Biopsy of her skin lesions revealed Sweet's syndrome. A work-up for malignancy eventually demonstrated a pelvic mass and carcinomatosis, and a diagnosis of advanced-stage papillary serous ovarian carcinoma was subsequently made. In postmenopausal women who present with Sweet's syndrome, a comprehensive evaluation for malignancy is indicated. In women with a known diagnosis of cancer, Sweet's syndrome may manifest in the detection of persistent or recurrent disease.
Sweet's syndrome is a condition characterized by fever, neutrophilia, tender, erythematous skin lesions, and a dermal infiltrate consisting predominantly of mature neutrophils on histologic analysis [1,2]. It is a reactive phenomenon and is commonly a cutaneous marker of systemic disease. Initially described by Dr. Robert Sweet in 1964, Sweet's syndrome has been associated with infection, pregnancy, autoimmune diseases, drugs, vaccines, and malignancies [3]. Malignancy-associated Sweet's syndrome (MASS) accounts for approximately 20% of cases and is observed most often in individuals diagnosed with acute myelogenous leukemia. It may precede the initial diagnosis of a cancer and thus herald the presence of malignancy or manifest in the detection of persistent or recurrent disease [3].
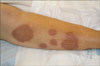
A 56-year-old female presented to her primary care physician with fevers, abdominal pain and tender, scarlet lesions on her extremities. The skin lesions and fever, which developed in the 4 weeks prior to presentation, preceded the abdominal pain. The lesions were irregular, sharply-bordered coalescing plaques that varied in size (Fig. 1). The patient was noted to be anemic (hemoglobin, 8.3 mg/dL) and to have a neutrophilia (polymorphonuclear leukocytes, >12,000/mm3). Biopsy of her skin lesions revealed a dense dermal infiltrate of neutrophils, confirming a diagnosis of Sweet's syndrome. An elevated serum creatinine (1.9 mg/dL) prompted an intravenous pyelogram study which revealed severe right hydronephrosis and hydroureter. She underwent a retrograde pyelogram and cystoscopy with ureteral stent placement and was placed on a one-week oral high-dose corticosteroid regimen. However, she developed worsening abdominal symptoms and her dermal lesions persisted. A CT scan revealed a 7×4.4 cm right-sided soft tissue pelvic mass causing right ureteral compression along with evidence of carcinomatosis and intrahepatic lesions concerning for metastatic disease. A serum CA-125 was 764 units/mL. She was transferred to our institution for continued care.
At the time of surgery through a midline, vertical abdominal incision, an 8×5 cm right ovarian mass was observed fixed to the right ureter and ileocecal mesentery. Because of the extensive tumoral involvement in the pelvis, a radical hysterectomy, bilateral salpingo-oophorectomy, right hypogastric artery ligation, partial resection of right pelvic ureter with a neoureterocystotomy/psoas hitch and ureteral stenting, ileocecal resection with functional end-to-end anastomosis were indicated. Liver biopsies and an omentectomy were also performed when it became clear that this was likely a primary ovarian malignancy. The patient was optimally cytoreduced to <1 cm residual disease and final pathology revealed a stage IV poorly differentiated ovarian carcinoma (due to the liver metastases).
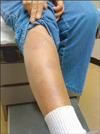
Postoperatively, the patient did well. Her foley catheter was removed 10 days after surgery (a retrograde cystogram revealed no leaking). She was discharged home on postoperative day 12 in good condition. Corticosteroids were briefly discontinued for 2 weeks to allow for healing from the surgery, but restarted and continued for 4 additional weeks. High-dose prednisone was prescribed and then tapered during the last 2 weeks of therapy. After treatment with six cycles of intravenous carboplatin/paclitaxel, she has experienced a complete response and her skin lesions have largely faded or disappeared (Fig. 2).
To our knowledge, this report of Sweet's syndrome associated with ovarian carcinoma is only the second described in the literature, the first reported in 1983, suggesting that ovarian carcinoma is among the growing number of cancers in which Sweet's syndrome may occur as a paraneoplastic process [1,5]. After performing a PubMed search for associations of this syndrome with other gynecologic malignancies using the terms, Sweet's syndrome, ovarian cancer, uterine cancer, cervical cancer, fallopian tube cancer, and peritoneal cancer, we identified only 1 case of ovarian cancer and 2 cases of cervical cancer associated with Sweet's syndrome. In all cases Sweet's syndrome was the first sign of either a new gynecologic malignancy or recurrence [4-7]. Although rare, it is critical that gynecologists and gynecologic oncologists are aware of the possible paraneoplastic and cutaneous manifestations of malignancy that may herald an underlying gynecologic cancer. In this case, Sweet's syndrome developed as a presenting feature of ovarian carcinoma, although it may precede the diagnosis of malignancy by months.
Twenty percent of all cases are MASS, and approximately 60% precede or occur as a presenting feature of malignancy [8]. Although most commonly associated with blood cancers, Sweet's syndrome also has been associated with several solid tumors, most commonly carcinomas of the genitourinary organs (37%), breast (23%), and gastrointestinal tract (17%) [1,8]. The pathogenesis of Sweet's syndrome may be multi-factorial but still remains to be definitively established.
The gold standard treatment of Sweet's syndrome is a course of systemic corticosteroids [1]. In most cases, the skin lesions resolve after steroid therapy, although as was the case with our patient with MASS, eradication of the tumor by surgical extirpation and/or adjuvant treatment may be required for skin lesions to completely resolve. Moreover, recurrence may follow either spontaneous remission or therapy-induced clinical resolution. We shall perform serial surveillance for progressive or recurrent disease in our patient with clinical exam of her skin for MASS lesions, pelvic exams, serum CA-125 and serial imaging. It is possible that if the patient's MASS lesions recur, they may herald the presence of recurrent cancer and allow for earlier detection and initiation of second line therapy.
Figures and Tables
References
1. Cohen PR, Kurzrock R. Sweet's syndrome: a review of current treatment options. Am J Clin Dermatol. 2002. 3:117–131.
2. Cohen PR, Kurzrock R. Sweet's syndrome revisited: a review of disease concepts. Int J Dermatol. 2003. 42:761–778.
3. Cohen PR, Holder WR, Tucker SB, Kono S, Kurzrock R. Sweet syndrome in patients with solid tumors. Cancer. 1993. 72:2723–2731.
4. Culp L, Crowder S, Hatch S. A rare association of Sweet's syndrome with cervical cancer. Gynecol Oncol. 2004. 95:396–399.
5. Nguyen KQ, Hurst CG, Pierson DL, Rodman OG. Sweet's syndrome and ovarian carcinoma. Cutis. 1983. 32:152–154.
6. Serirat O, Thaipisuttikul Y. Sweet's syndrome associated with Mycobacterium tuberculosis and cervical cancer: a case report. J Med Assoc Thai. 2011. 94:Suppl 2. S119–S122.
7. Loehberg L, Simon M. Sweets syndrome: a rare first cutaneous diagnostic sign in cervical cancer. Eur J Dermatol. 2009. 19:87–88.
8. Cohen PR. Sweet's syndrome: a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007. 2:34.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download