Abstract
Objective
The purpose of this study was to determine the predictive factors for residual/recurrent disease and to analyze the timing for Pap smears and human papillomavirus (HPV) testing during follow-up after loop electrosurgical excision procedure (LEEP) for cervical intraepithelial neoplasia (CIN) 2 or worse.
Methods
We retrospectively analyzed 183 patients (mean age, 39.3 years) with CIN 2/3 who were treated with LEEP. Post-LEEP follow-up was performed by Pap smear and HPV hybrid capture2 (HC2) testing. The definition of persistent/recurrent disease was biopsy-proven CIN 2 or worse.
Results
Among 183 patients, punch biopsies were CIN 2 in 31 (16.9%) and CIN 3 in 152 (83.1%). HPV HC2 tests before LEEP were positive in 170 (95.5%) of 178 patients. During follow-up, 12 patients (6.6%) had residual/recurrent CIN 2+. LEEP margin status was a significant predictive factor for persistent/recurrent disease. Other factors such as age, HPV HC2 viral load (≥100 relative light units), and HPV typing (type 16/18 vs. other types) did not predict recurrence. Early HPV HC2 testing at 3 months after LEEP detected all cases of residual/recurrent disease. The sensitivity and negative predictive value of the HPV HC2 test for residual/recurrent disease were both 100% at 3 and 6 months.
Conclusion
Margin involvement in conization specimens was a significant factor predicting residual/recurrent disease after LEEP. HPV test results at 3 and 6 months after treatment were comparable. Early 3-month follow-up testing after LEEP can offer timely information about residual/recurrent disease and alleviate patient anxiety early about treatment failure.
Numerous studies have revealed that conservative treatment for high-grade cervical intraepithelial neoplasia (CIN) is effective for preventing the development of invasive cervical cancer [1,2]. Since the early 1990s, the loop electrosurgical excision procedure (LEEP) has been a popular modality for local treatment of CIN because of its many advantages over cryosurgery and laser vaporization [3,4]. Post-treatment CIN rates of 5-15% have been reported following CIN excision using LEEP [5]. Long-term follow-up after local treatment of CIN is mandatory due to the late occurrence of cervical cancer over a period of 20 years [6-10]. Early detection of treatment failure is vital.
The optimal method for confirming the clearance of the neoplastic process by LEEP is controversial. Historically, cervical cytology was the main tool for detecting residual/recurrent dysplasia during follow-up. Recently, human papillomavirus (HPV) DNA testing after conization has been used in numerous studies and is a preferred tool with several advantages over cytology [11,12]. The negative conversion of high-risk (HR) HPV after conization in patients with positive HR HPV before treatment usually occurs during follow-up and suggests the success of conization [13,14]. Nevertheless, the timing for follow-up after conization is still unclear and ranges from 1 to 6 months after conization [12,13,15,16].
The purpose of this study was to determine the predictive factors for residual/recurrent disease and to analyze the timing for Pap smears and HPV testing in order to detect residual/recurrent disease during follow-up after LEEP for CIN 2 or higher.
A retrospective analysis was used to examine women who underwent LEEP for CIN at Soonchunhyang University Bucheon Hospital, Korea from January 2005 to June 2008. In total, 459 patients underwent LEEP conization for CIN during the study period. The patients underwent an examination at 4-6 weeks postoperatively, at 3, 6, 12, 18, and 24 months during the first 2 years and yearly thereafter. At each visit, except the postoperative visit at 4-6 weeks, patients received a liquid-based cytology test, an HPV hybrid capture2 (HC2) test, colposcopic assessment, and if indicated, colposcopy-directed punch biopsy of the cervix. If CIN 2/3 was identified at the margins of a diagnostic excisional procedure or in an endocervical cytology obtained immediately after LEEP, we followed the 2006 American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines. Patients were reassessed using cytology with endocervical sampling and HPV testing at 3-6 months post-treatment as in patients with negative margins.
Inclusion criteria were CIN 2 or 3 on a colposcopic punch biopsy and/or excised specimen, adequate 3- and 6month follow-up after LEEP without a hysterectomy, and an HPV HC2 test and/or HPV DNA chip test before and after LEEP. Of the 459 patients, 59 (12.9%) underwent a hysterectomy during the study period, primarily due to invasive cancer, and 108 (23.5%) had no or only one follow-up visit after LEEP. In total, 29 patients with CIN 1 or microinvasion were also excluded. Eighty patients were excluded due to irregular follow-up after LEEP. Finally, 183 patients satisfied the inclusion criteria. Epidemiological data, pathological reports, high-risk (HR)-HPV test results, and follow-up data from the medical records were reviewed.
We examined age, cytology, HR-HPV HC2 viral load, HPV DNA type, and margin involvement status as possible predictive factors for residual/recurrent disease. Age was divided into young (<50 years) and old (≥50 years) age groups. HPV load was classified as high (≥100 relative light units, RLU) and low (<100 RLU). HPV genotyping was divided into two categories: HPV type 16 and/or 18 and other HPV types, including negative cases. For statistical analyses, cases of multiple HPV infection including type 16 or 18 were categorized in the HPV 16/18 group.
The definition of residual/recurrent disease during follow-up was biopsy-proven CIN 2 or higher, using punch or re-LEEP specimens. Women with two consecutive negative Pap cytology smears and normal colposcopy findings were considered negative for a residual/recurrent lesion regardless of the HPV HC2 result.
The liquid-based preparation test (ThinPrep, Hologic Inc., Bedford, MA, USA) was performed using a soft plastic spatula and endocervical cytobrush (Medland, Seoul, Korea). All specimens were stained using the Papanicolaou method. Final cytological diagnosis was achieved using the Bethesda System. Diagnoses were classified as normal or inflammatory, atypical squamous cells (ASC), low-grade squamous intraepithelial lesion (LSIL), or high-grade squamous intraepithelial lesion (HSIL). Cytology was divided into two groups: the low-grade group included normal, atypical squamous cells of undetermined significance (ASCUS), atypical squamous cells that could not exclude high-grade SIL (ASC-H), and LSILs; the high-grade group consisted of HSILs or higher. During the follow-up, an abnormal cytological result was defined as any report of HSIL or higher.
Detailed procedures for detecting HR-HPV have been described previously [14,17]. Briefly, HC2 assay samples were obtained using a cytobrush (Digene Cervical Sampler, Digene, Gaitherburg, MD, USA) during a second swab of the cervix and transferred to a vial containing Digene Specimen Transport Medium. The samples were tested for 13 oncogenic genotypes (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68), and the results were classified as positive at a RLU/cutoff ratio of ≥1 pg/mL. Light measurements were quantified using a luminometer and are expressed as the ratio between the RLU of a clinical sample and that of the positive control (PC). The luminescence of a specimen was compared with that of a 1.0 pg/mL HPV-16 cutoff standard. In most previous studies, HC2 has shown a high sensitivity and negative predictive value (90-95%) with this cutoff [18].
A commercial HPV DNA chip (MyHPV Chip; Mygene Co., Seoul, Korea) was also used for HPV genotyping. The HPV chip can detect 24 type-specific HPVs (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 54, 56, 58, 59, 66, and 68) and eight HPVs in the low-risk group (6, 11, 34, 40, 42, 43, 44, and 70). Target HPV DNA was amplified by polymerase chain reaction (PCR) with specific primers (HPV and human β globulin) using conditions provided by Mygene (Seoul, Korea), and was labeled using Cy5-deoxyuridine triphosphate (NEN Life Science Products Inc., Boston, MA, USA). The PCR product was hybridized on the chip at 40℃ for 2 hours, and the chip was washed with 3×SSPE (3.0 M sodium chloride, 0.2 M sodium hydrogen phosphate, 0.02 M EDTA, pH 7.4). Hybridized signals were visualized using a DNA chip scanner (ScanArray Lite, GSI Lumonics Inc., Ottawa, ON, Canada).
The cervix was exposed using an adapted speculum that allowed smoke evacuation. Local anesthesia was induced with an injection of 2% lidocaine plus epinephrine at the 3, 6, 9, and 12 o'clock positions of the cervix, using a 31 G dental needle. The electrosurgical procedure was performed with a high-frequency electrical generator. The loop was selected according to the size of the area to be excised. When the exocervical lesion was too large to be accommodated by a single sweep, it was excised with two or more systematic sweeps; to establish the true excisional margins, the specimens were gathered into their original anatomical shape by the operator before being sent to the pathology laboratory. The excised wound base was cauterized by ball diathermy. When an endocervical extension was suspected, an additional apical specimen was taken using a small wire-loop electrode. The 12 o'clock position was marked by cutting the excised specimen. The pathological report described the severity of disease (CIN 2/3), marginal status (exocervical or endocervical; clear or involved), and glandular involvement (present or absent), according to World Health Organization criteria.
Statistical analyses were performed using the SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA). Data were analyzed using Fisher' s exact test and logistic regression analysis. Negative conversion of HPV HC2 after LEEP was analyzed using McNemar's test. Agreement between 3- and 6-month HPV HC2 and Pap tests were analyzed using κ statistics, with values between 0 and 0.20 indicating poor agreement, 0.21-0.40 fair agreement, 0.41-0.60 moderate agreement, 0.61-0.80 good agreement, and 0.81-1.00 very good agreement. All tests were two-sided, and the level of significance was set at p<0.05.
The average age of the 183 patients was 39.3±8.7 years (range, 22 to 73 years) (Table 1). The average follow-up was 25.3±13.3 months (range, 4 to 60 months). The first follow-up occurred at an average of 3.3 months; and the second, at 6.7 months. During follow-up, 12 (6.6%) of the 183 patients had residual/recurrent lesions, including CIN 2 (2, 16.7%), CIN 3 (9, 75%), and microinvasion (1, 8.3%). The mean lag time between LEEP and the recurrent disease diagnosis was 14.0 months (range, 4 to 41 months).
The Pap test before LEEP showed that 94 (51.4%) patients had low-grade lesions (8 normal, 68 ASCUS, 18 LSIL) and 73 (43.7%) had high-grade lesions (70 HSIL, 3 cancers). Thirty-one patients (16.9%) had CIN 2, and 152 (83.1%) had CIN 3, as determined from a punch or LEEP specimen. The average preconization HPV HC2 viral load was 476.6±679.4 RLU. In total, 170 (92.9%) of the 178 patients were positive for the HR-HPV HC2 test before LEEP. Eight patients who had negative HR-HPV HC2 test results were also negative for the same test during follow-up. Five patients who did not undergo the HPV HC2 test before LEEP had negative test results during follow-up. Of the 170 patients with positive HPC HC2 tests, 122 (71.8%) were negative for conversion at 3 months based on the HPV HC2 test (p<0.001). The HPV genotype analysis showed that HPV-16 was the most common type, present in 77 (42.1%) of 175 patients who had HPV genotyping before LEEP (Table 1). A negative margin was observed in 135 (73.8%), and 48 (26.3%) patients had an involved margin.
Twelve (6.6%) of the 183 patients had residual/recurrent lesions during follow-up. Residual or recurrent lesions included CIN 2 (2, 16.7%), CIN 3 (9, 75%), and microinvasion (1, 8.3%) (Table 1). Table 2 shows the risk factors for residual/recurrent disease, analyzed using logistic regression. Age (cutoff, 50 years), preoperative cytology, HPV HC2 viral load (cutoff, 100 RLU), and HPV genotype (type 16 plus 18 vs. other types and negative cases) were not relevant predictors of residual/recurrent disease. Margin status was a significant prediction factor for residual/recurrent disease (odds ratio [OR], 39.079; 95% confidence interval [CI], 4.399 to 347.184; p=0.001) (Table 2).
Of 168 patients without residual/recurrent disease, 134 (79.8%) were negative on the HPV HC2 test, and the remaining 34 (20.2%) were still positive when tested at 3 months after LEEP (Table 3). All 12 patients with residual/recurrent disease had positive HPV HC2 test results at 3 months. We analyzed the diagnostic accuracy of the HPV HC2 test and cytology at 3 and 6 months. The sensitivity of the HPV HC2 test for detecting residual/recurrent disease was 100% at 3 and 6 months. The specificity, diagnostic accuracy, and likelihood ratio of a positive result for the HPV HC2 test were similar at 3 and 6 months (Table 4). Pap cytology showed a lower sensitivity and higher specificity, diagnostic accuracy, and likelihood ratio of a positive result compared with the HPV test (Table 4). In terms of agreement between the 3- and 6-month HPV HC2 test and Pap cytology, the kappa score was 0.728 (95% CI, 0.599 to 0.857) for the HPV HC2 test and 0.727 (95% CI, 0.984 to 0.490) for Pap cytology, showing good agreement.
Treatment failure is an important concern after conservative treatment of a high-grade cervical lesion. In our study, 12 (6.6%) of 183 patients developed residual/recurrent disease, which was similar with data reported previously [19-22]. Although the success rate of conservative treatment for CIN 2/3 by LEEP is high, the risk of CIN 2+ is much greater than that in the screening population, and late recurrent or invasive disease may develop. Soutter et al. [9] reported that the cumulative rate of invasion 8 years after treatment was 5.8 per 1,000 women. Long-term follow-up after local CIN treatment is mandatory due to the late occurrence of cervical cancer over 20 years [7,10], and has been recommended by the ASCCP consensus guidelines [15].
Despite the importance of early detection of treatment failure, follow-up after conservative treatment of high-grade CIN has not yet been standardized and varies in term of timing, intervals, and methods. According to the ASCCP consensus guidelines, acceptable post-treatment management options for women with CIN 2/3 include HPV DNA testing at 6 to 12 months. Follow-up using cytology alone or in combination with a colposcopy at 6 months is also acceptable [23]. Several investigators have analyzed the sensitivity and specificity of HPV DNA testing compared with follow-up cytology to more accurately detect residual/recurrent disease after treatment [11,24,25]. HPV testing was found to be more sensitive than follow-up cytology, with comparable specificity [12,25]. Women who are HPV-positive after surgery are at higher risk for treatment failure; specifically, there is a lower probability of a negative HPV test eliminating the risk of residual/recurrent disease [12,20]. Negative conversion from a positive HR-HPV infection in patients with CIN 2/3 occurred earlier in a subsequent hysterectomy specimen at 3-6 weeks after conization treatment [26,27]. Jain et al. [26] reported that no residual lesion was detected in women with negative HPV test results at 6 weeks after conization (35 cases, 79 patients; 100% negative predictive value), irrespective of margin involvement in the subsequent hysterectomy specimen. This finding that viral clearance at follow-up after conization is significantly associated with the effectiveness of surgical treatment was reconfirmed by Cricca et al. [28]. Viral clearance at follow-up, suggesting treatment success, can occur in cases of margin involvement [28]. In the present study, no residual/recurrent disease was detected in the women with negative HPV HC2 test results at 3 months, irrespective of margin involvement, indicating 100% negative predictive value for residual/recurrent disease during follow-up; this finding is consistent with data reported previously [21,25,28]. Similar results were obtained for the 6-month HR-HPV HC2 test in our study (Table 4), which was consistent with Zielinski's report that the 6-month result does not differ significantly from the result at 3 months [29,30]. They reported that the sensitivity for predicting residual/recurrent CIN 2/3 and cervical cancer using combined cytology and HPV testing at 3 and 6 months post-treatment was 100%; the specificities were 76% and 81%, respectively; and the negative predictive value was 100%. High negative predictive value is important in this post-treatment population, because women who test negative can return to a normal screening schedule. As shown in Table 5, post-treatment HPV HC2 testing had similar sensitivity and specificity for detecting residual/recurrent CIN grade ≥2 irrespective of the time between treatment and the first HPV test, which was 3-6 months [12,19-22,25]. Some investigators advocate that when post-treatment HR-HPV DNA is absent at 3 to 6 months after conization, especially in patients with a negative cone margin, follow-up can be relaxed, and the patient can resume general population screening [21,26]. Previous research investigating the psychological impact of HPV infection on routine cervical cancer screening found that testing positive for HPV had an adverse psychological impact, with increased anxiety, distress, and concern about sexual relationships [31]. Cervical conization such as LEEP is perceived as distressing and as more painful than a diagnostic colposcopic examination [32]. HPV testing at 3 months rather than 6 months after treatment can alleviate patient anxiety and allow an earlier return to normal life. Coupe et al. [33] advocated HPV testing only at the first follow-up date to reduce costs and patient burden.
The well-known risk factors for CIN residual/recurrent disease after conservative treatment are age, parity, cytological grade, lesion grade, preoperative and follow-up HPV viral load, HPV genotype, and cone margin involvement. Sarian et al. [19] reported that women older than 35 years had a significantly higher risk for persistent infection following LEEP, indicating older age as a predictive factor for a residual lesion or increased risk for disease recurrence. Older age at conization is a previously unrecognized risk factor for recurrence, as reported by Verguts et al. [21]. In contrast, some studies have shown that age is not related to the persistence of HPV infection after treatment, and this is consistent with our results [34,35]. Cytological grade before LEEP was also not a significant factor for residual disease or recurrence in the present study, which is consistent with previous reports [20,36].
High pre-conization HR-HPV viral load as a predictor of residual/recurrent disease has been studied by several investigators. In our study, a high HR-HPV HC2 viral load, using a cutoff of ≥100 RLU, was not a significant risk factor for predicting residual/recurrent disease after LEEP (p=0.074; OR, 7.342; 95% CI, 0.827 to 65.200), in agreement with a previous report [37]. Alonso et al. [12] reported contradictory results; however, they used RLU/PC of 1,000 as the cutoff value for a high viral load. Kang et al. [20], using the area under the receiver operating characteristics curve for HR-HPV HC2 viral load using variable cutoffs (1, 10, 100, and 1,000 RLU) in 600 patients, showed that HPV viral load was not associated with residual/recurrence (p=0.466), which is consistent with our results. A review of published results demonstrated that the definition of a high HR-HPV viral load has been arbitrary and needs to be standardized in the near future.
Since the first report by Gok et al. [38] that preconization infection with HPV-16 increases the risk for recurrent disease during CIN 3 treatment, further studies have shown that preconization infection with several other HPV genotypes is also a risk factor for residual/recurrent disease, which contradicts our results. In our study, HPV genotypes were grouped as type 16 plus 18 versus other high-risk types and negative cases. Some other reports have considered each genotype independently and showed that preconization HPV-16 was a risk factor for developing residual/recurrent CIN 2/3 [20,29,39]. Recent data showed that the presence of HPV-16, -18, -33, or -45, as well as multiple HPV types, before LEEP is associated with higher rates of residual/recurrent disease after LEEP [39]. Few studies have reported HPV genotype testing during follow-up after LEEP. Kang et al. [20] suggested that persistent infection with the same HR-HPV genotype, particularly HPV-16 and HPV-18, should be regarded as a risk factor for developing residual/recurrent CIN 2/3. Similarly, Kreimer et al. [22] reported that HPV-16 positivity in samples collected 6 months post-LEEP was associated with an increase in the 2-year absolute risk for CIN 2+ to 37%, twice that associated with HPV-18 (18.5%) and more than three times that associated with other oncogenic types (10.8%). This result indicates that the HPV genotype should be considered in post-treatment monitoring policies, as advocated by Gok et al. [38]. We could not report these results because we did not include HR-HPV genotyping during follow-up.
Many studies clearly demonstrate that margin involvement significantly increases the risk for residual/recurrent disease [6,25,40]. A meta-analysis of incomplete CIN excision showed that high-grade post-treatment CIN occurred in 597 (18%) of 3,335 women who had margin involvement versus 318 (3%) of 12,493 women who had complete excision [6]. We found a similar result, as residual/recurrent CIN 2+ occurred in 10 (20.8%) of 48 patients who had margin involvement versus 2 (1.5%) of 135 who received complete excision. Our study further supports previous findings showing that the risk for residual/recurrent disease is not negligible, even when margins are negative [6,20,41]. The reasons for recurrence after complete excision may include multifocal lesions, inadequate specimens, and HPV DNA persistence [11,13]. A hysterectomy should be performed only when the surgeon is not confident that invasive disease more advanced than stage IA1 is absent [6].
Previous studies have shown that about one-fifth of patients with LEEP specimen margin involvement will have high-grade residual/recurrent CIN. Despite the high risk for residual/recurrence in cases of margin involvement, virtually all residual/recurrent patients can be detected by either HC2 or HPV genotype testing. If HPV genotyping during follow-up were to indicate HPV genotypes different from those present before LEEP, particularly for HPV-16 or HPV-18, the patient could be advised to return to normal screening for cervical cancer. Notably, one drawback of the HPV test after LEEP treatment is its low specificity. Especially in younger women, false-positive tests can result in a high referral rate for colposcopy, which may cause anxiety in patients.
The current routine follow-up procedure lacks randomized controlled trials to allow for a comparison among follow-up strategies and HPV detection methods. Ideally, a randomized controlled trial with a follow-up period of at least 5 years would allow researchers to compare different HPV tests with cytology to clarify the best strategies and the impacts of false-positive and false-negative findings. However, the number of studies with follow-up testing between 3 and 6 months after LEEP is limited [29].
Some limitations of this study include its retrospective study design, lack of follow-up for all treated patients, and lack of HPV genotype testing during follow-up. Nevertheless, the results are largely consistent with recently published data.
In conclusion, our results suggest that when the cone margin is positive and an HPV HC test is negative, the risk for residual/recurrent disease is negligible. HPV testing and Pap cytology between 3 and 6 months after treatment showed comparable results. Therefore, an early follow-up test at 3 months after LEEP can offer timely information about residual or recurrent disease and quickly alleviate patient anxiety about treatment failure, thus helping patients return to a normal screening schedule.
Figures and Tables
Table 1
Patient characteristics (n=183)
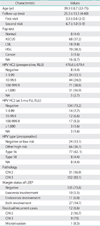
Values are presented as mean±SD (range) or number (%).
ASCUS, atypical squamous cells of undetermined significance; CIN, cervical intraepithelial neoplasia; HC2, hybrid capture 2; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LEEP, loop electrosurgical excision procedure; LSIL, low-grade squamous intraepithelial lesion; NA, not available; RLU, relative light units.
Table 3
HPV test results at the first 3-month follow-up visit according to margin status and residual/recurrent disease

References
1. McCredie MR, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008. 9:425–434.
2. Pinto AP, Crum CP. Natural history of cervical neoplasia: defining progression and its consequence. Clin Obstet Gynecol. 2000. 43:352–362.
3. Prendiville W, Cullimore J, Norman S. Large loop excision of the transformation zone (LLETZ): a new method of management for women with cervical intraepithelial neoplasia. Br J Obstet Gynaecol. 1989. 96:1054–1060.
4. Lindeque BG. Management of cervical premalignant lesions. Best Pract Res Clin Obstet Gynaecol. 2005. 19:545–561.
5. Mitchell MF, Tortolero-Luna G, Cook E, Whittaker L, Rhodes-Morris H, Silva E. A randomized clinical trial of cryotherapy, laser vaporization, and loop electrosurgical excision for treatment of squamous intraepithelial lesions of the cervix. Obstet Gynecol. 1998. 92:737–744.
6. Ghaem-Maghami S, Sagi S, Majeed G, Soutter WP. Incomplete excision of cervical intraepithelial neoplasia and risk of treatment failure: a meta-analysis. Lancet Oncol. 2007. 8:985–993.
7. Soutter WP, Sasieni P, Panoskaltsis T. Long-term risk of invasive cervical cancer after treatment of squamous cervical intraepithelial neoplasia. Int J Cancer. 2006. 118:2048–2055.
8. Soutter WP. Invasive cancer after treatment of cervical intraepithelial neoplasia. Ann Acad Med Singapore. 1998. 27:722–724.
9. Soutter WP, de Barros Lopes A, Fletcher A, Monaghan JM, Duncan ID, Paraskevaidis E, et al. Invasive cervical cancer after conservative therapy for cervical intraepithelial neoplasia. Lancet. 1997. 349:978–980.
10. Strander B, Andersson-Ellstrom A, Milsom I, Sparen P. Long term risk of invasive cancer after treatment for cervical intraepithelial neoplasia grade 3: population based cohort study. BMJ. 2007. 335:1077.
11. Costa S, De Simone P, Venturoli S, Cricca M, Zerbini ML, Musiani M, et al. Factors predicting human papillomavirus clearance in cervical intraepithelial neoplasia lesions treated by conization. Gynecol Oncol. 2003. 90:358–365.
12. Alonso I, Torne A, Puig-Tintore LM, Esteve R, Quinto L, Campo E, et al. Pre- and post-conization high-risk HPV testing predicts residual/recurrent disease in patients treated for CIN 2-3. Gynecol Oncol. 2006. 103:631–636.
13. Nagai Y, Maehama T, Asato T, Kanazawa K. Persistence of human papillomavirus infection after therapeutic conization for CIN 3: is it an alarm for disease recurrence? Gynecol Oncol. 2000. 79:294–299.
14. Nam K, Chung S, Kim J, Jeon S, Bae D. Factors associated with HPV persistence after conization in patients with negative margins. J Gynecol Oncol. 2009. 20:91–95.
15. Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D, et al. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol. 2007. 197:340–345.
16. Heley S. Pap test update. Aust Fam Physician. 2007. 36:112–115.
17. Nam K, Chung S, Kwak J, Cha S, Kim J, Jeon S, et al. Random biopsy after colposcopy-directed biopsy improves the diagnosis of cervical intraepithelial neoplasia grade 2 or worse. J Low Genit Tract Dis. 2010. 14:346–351.
18. Clavel C, Cucherousset J, Lorenzato M, Caudroy S, Nou JM, Nazeyrollas P, et al. Negative human papillomavirus testing in normal smears selects a population at low risk for developing high-grade cervical lesions. Br J Cancer. 2004. 90:1803–1808.
19. Sarian LO, Derchain SF, Andrade LA, Tambascia J, Morais SS, Syrjanen KJ. HPV DNA test and Pap smear in detection of residual and recurrent disease following loop electrosurgical excision procedure of high-grade cervical intraepithelial neoplasia. Gynecol Oncol. 2004. 94:181–186.
20. Kang WD, Oh MJ, Kim SM, Nam JH, Park CS, Choi HS. Significance of human papillomavirus genotyping with high-grade cervical intraepithelial neoplasia treated by a loop electrosurgical excision procedure. Am J Obstet Gynecol. 2010. 203:72.e1–72.e6.
21. Verguts J, Bronselaer B, Donders G, Arbyn M, Van Eldere J, Drijkoningen M, et al. Prediction of recurrence after treatment for high-grade cervical intraepithelial neoplasia: the role of human papillomavirus testing and age at conisation. BJOG. 2006. 113:1303–1307.
22. Kreimer AR, Guido RS, Solomon D, Schiffman M, Wacholder S, Jeronimo J, et al. Human papillomavirus testing following loop electrosurgical excision procedure identifies women at risk for posttreatment cervical intraepithelial neoplasia grade 2 or 3 disease. Cancer Epidemiol Biomarkers Prev. 2006. 15:908–914.
23. Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D, et al. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. J Low Genit Tract Dis. 2007. 11:223–239.
24. Sarian LO, Derchain SF, Pitta Dda R, Morais SS, Rabelo-Santos SH. Factors associated with HPV persistence after treatment for high-grade cervical intra-epithelial neoplasia with large loop excision of the transformation zone (LLETZ). J Clin Virol. 2004. 31:270–274.
25. Houfflin Debarge V, Collinet P, Vinatier D, Ego A, Dewilde A, Boman F, et al. Value of human papillomavirus testing after conization by loop electrosurgical excision for high-grade squamous intraepithelial lesions. Gynecol Oncol. 2003. 90:587–592.
26. Jain S, Tseng CJ, Horng SG, Soong YK, Pao CC. Negative predictive value of human papillomavirus test following conization of the cervix uteri. Gynecol Oncol. 2001. 82:177–180.
27. Lin CT, Tseng CJ, Lai CH, Hsueh S, Huang KG, Huang HJ, et al. Value of human papillomavirus deoxyribonucleic acid testing after conization in the prediction of residual disease in the subsequent hysterectomy specimen. Am J Obstet Gynecol. 2001. 184:940–945.
28. Cricca M, Venturoli S, Morselli-Labate AM, Costa S, Santini D, Ambretti S, et al. HPV DNA patterns and disease implications in the follow-up of patients treated for HPV16 high-grade carcinoma in situ. J Med Virol. 2006. 78:494–500.
29. Zielinski GD, Rozendaal L, Voorhorst FJ, Berkhof J, Snijders PJ, Risse EJ, et al. HPV testing can reduce the number of follow-up visits in women treated for cervical intraepithelial neoplasia grade 3. Gynecol Oncol. 2003. 91:67–73.
30. Chua KL, Hjerpe A. Human papillomavirus analysis as a prognostic marker following conization of the cervix uteri. Gynecol Oncol. 1997. 66:108–113.
31. McCaffery K, Waller J, Forrest S, Cadman L, Szarewski A, Wardle J. Testing positive for human papillomavirus in routine cervical screening: examination of psychosocial impact. BJOG. 2004. 111:1437–1443.
32. Kola S, Walsh JC. Patients' psychological reactions to colposcopy and LLETZ treatment for cervical intraepithelial neoplasia. Eur J Obstet Gynecol Reprod Biol. 2009. 146:96–99.
33. Coupe VM, Berkhof J, Verheijen RH, Meijer CJ. Cost-effectiveness of human papillomavirus testing after treatment for cervical intraepithelial neoplasia. BJOG. 2007. 114:416–424.
34. Dalstein V, Riethmuller D, Pretet JL, Le Bail Carval K, Sautiere JL, Carbillet JP, et al. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: a longitudinal French cohort study. Int J Cancer. 2003. 106:396–403.
35. Franco EL, Villa LL, Sobrinho JP, Prado JM, Rousseau MC, Desy M, et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis. 1999. 180:1415–1423.
36. Park JY, Lee KH, Dong SM, Kang S, Park SY, Seo SS. The association of pre-conization high-risk HPV load and the persistence of HPV infection and persistence/recurrence of cervical intraepithelial neoplasia after conization. Gynecol Oncol. 2008. 108:549–554.
37. Bae JH, Kim CJ, Park TC, Namkoong SE, Park JS. Persistence of human papillomavirus as a predictor for treatment failure after loop electrosurgical excision procedure. Int J Gynecol Cancer. 2007. 17:1271–1277.
38. Gok M, Coupe VM, Berkhof J, Verheijen RH, Helmerhorst TJ, Hogewoning CJ, et al. HPV16 and increased risk of recurrence after treatment for CIN. Gynecol Oncol. 2007. 104:273–275.
39. Wu D, Zheng Y, Chen W, Guo C, Yu J, Chen G, et al. Prediction of residual/recurrent disease by HPV genotype after loop excision procedure for high-grade cervical intraepithelial neoplasia with negative margins. Aust N Z J Obstet Gynaecol. 2011. 51:114–118.
40. Paraskevaidis E, Kalantaridou SN, Paschopoulos M, Zikopoulos K, Diakomanolis E, Dalkalitsis N, et al. Factors affecting outcome after incomplete excision of cervical intraepithelial neoplasia. Eur J Gynaecol Oncol. 2003. 24:541–543.
41. Paraskevaidis E, Lolis ED, Koliopoulos G, Alamanos Y, Fotiou S, Kitchener HC. Cervical intraepithelial neoplasia outcomes after large loop excision with clear margins. Obstet Gynecol. 2000. 95:828–831.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download