This article has been corrected. See "Journal correction: Radiation therapy with chemotherapy for patients with cervical cancer and supraclavicular lymph node involvement" in Volume 23 on page 293.
Abstract
Objective
We wanted to evaluate the outcomes of cervical cancer patients with supraclavicular lymph node (SCLN) involvement and who received radiation therapy (RT) combined with chemotherapy.
Methods
From August 2001 to April 2009, nine cervical cancer patients with SCLN involvement were treated by RT and cisplatin-based chemotherapy. Most of the patients (8/9, 88.9%) also had a positive para-aortic lymph node (PALN). The RT field was designed to include the whole pelvis, the involved PALNs and the SCLN area. The median SCLN RT dose was 66.6 Gy (range, 60 to 70 Gy).
Results
The median follow-up period was 61 months (range, 13 to 98 months). The 3- and 5-year overall survival rates were 66.7% and 55.6%, respectively and the 3- and 5-year progression-free survival rates were 66.7% and 44.4%, respectively. The acute hematologic toxicities according to the criteria of Radiation Therapy of Oncology Group (RTOG) were G1/2 leucopenia in 3 (33.3%), G3/4 leukopenia in 6 (66.7%), G1/2 anemia in 7 (77.8%), G3 anemia in 1 (11.1%), G2 thrombocytopenia in 2 (22.2%), and G3/4 thrombocytopenia in 2 (22.2%). Within 6 months after RT, most of the patients (5/6, 83.3%) recovered from the G3/4 leukopenia, except for 1 patient who received chemotherapy after completing RT due to subsequent bone metastasis.
The most important prognostic factor for cervical cancer is the extent of disease at the time of diagnosis. A decrease of survival has been reported for patients with advanced stage cervical cancer. The reported 5-year overall survival (OS) rates for the advanced stages are stage IIB (65%), stage III (45%), stage IVA (20%), and stage IVB (5%) [1]. For locally advanced cervical cancer, concurrent chemoradiation therapy (CCRT) is the standard treatment. The Radiation Therapy Oncology Group (RTOG) 90-01 reported a 5-year OS rate of 59% for International Federation of Gynecology and Obstetrics (FIGO) stage III or IVA patients who were treated with CCRT [2]. There have been several reports on the treatment of cervical cancer with para-aortic lymph node (PALN) involvement, which responds well to CCRT. For cervical cancer with supraclavicular lymph node (SCLN) involvement, radiation therapy (RT) is not widely performed due to the relative rarity of this condition and the poor prognosis. However, there have been few reports of the role of CCRT for cervical cancer with SCLN involvement. Recently published study [3] suggesting the role of CCRT in cervical cancer patients with SCLN involvement report that patients who had received RT to pelvis, PALN and SCLN with simultaneous chemotherapy showed a long term survival. There is also a report indicating that an adequate dose of RT to the SCLN area may produce a survival advantage for cervical cancer patients with SCLN involvement [4]. However, the impact of CCRT on the SCLNs involved at the time of the initial management still remains unknown. The aim of this study was to retrospectively evaluate the treatment outcomes for cervical cancer patients with SCLN involvement at the initial presentation and who were treated by CCRT.
Between April 2001 and April 2009, 1,077 cervix cancer patients (of any stage) were seen at Gachon University Gil Hospital. Among them, 166 cervix cancer patients were treated by CCRT, with or without surgery. The CCRT alone was performed in 100 cervix cancer patients. The total 17 patients, with SCLN involvement, were presented during the study period. Among them, 11 patients had SCLN involvement at the initial presentation whereas the other 6 patients developed SCLN involvement during follow-up after treatment. The CCRT to the SCLN was performed in 8 of 11 patients with SCLN involvement at the initial presentation. One out of 8 patients who received CCRT to the SCLN was excluded from this study because of mediastinum and bone metastasis at the initial presentation. The reason for no treatment to the SCLN in the other 3 patients, with SCLN involvement at the initial presentation, was that they had other metastatic lesions (bone, lung). These 3 patients received CCRT to the pelvis only. In 6 relapsed patients in SCLN, treated with CCRT or RT to the SCLN, 4 patients had other metastatic lesions (bone, lung, etc).
We retrospectively reviewed the treatment outcomes of 7 cervical cancer patients with SCLN involvement treated by CCRT between April 2001 and April 2009 at our hospital. The inclusion criteria for CCRT were cervical cancer patients with SCLN involvement with or without PALN involvement diagnosed based on biopsy or imaging studies. These patients had no other evidence of metastatic disease (lung, liver, bone, mediastinum, etc.) other than to the PALN and SCLN, and had no specific medical contraindication to the administration of chemotherapy. All of the 7 patients had SCLN involvement at the time of initial presentation. For cervical cancer patients suspected of having SCLN involvement, CT scanning was used to determine the resectability. For making the diagnosis of SCLN involvement, ultrasound-guided biopsy was performed in 5 patients (71%) including patients with SCLN recurrence. The pathological diagnoses were metastatic squamous cell carcinoma (SCC) and this was consistent with the pathology of the primary cervical cancer. In the other 2 patients (29%), the diagnosis of SCLN involvement was confirmed by using chest CT and PET.
The histological typing was evaluated according to the criteria of the World Health Organization (WHO) International Histological Classification of Tumors. Six to 12 weeks after treatment completion, the patients underwent a CT scan or PET and a tumor marker study (SCC antigen, SCC-Ag) for routine follow-up. The failures were defined as local, regional or distant. A local failure was defined as any progressive tumor disease at the primary sites (pelvis, PALN, and SCLN) and the tumor lesion within the irradiated field (in-field) following RT. Locoregional failure was defined as failure at either local (in-field) or regional sites. The regional sites denote nodal or visceral regions contiguous with the primary site and outside the original RT field. Distant failure refers to the appearance of recurrence in distant sites representing hematogenous dissemination (bone, lung, and liver) outside the treated area. The toxicity experienced during adjuvant therapy was evaluated using the RTOG/European Organisation for Research and Treatment of Cancer (EORTC) toxicity criteria.
All 7 patients underwent curative RT with concomitant chemotherapy for cervical cancer with SCLN involvement. Among the 7 patients with initial SCLN involvement, three patients (43%) received RT to the whole pelvis, PALN and SCLN areas simultaneously and the other 4 patients (57%) received sequential SCLN RT after whole pelvis and PALN RT. One patient was treated by radical hysterectomy because of excessive vaginal bleeding at the initial visit. The RT field was designed to include the whole pelvis, the involved the PALN, including the elective area of the PALNs and the SCLN area. RT was delivered to the planned target volume by using the three-dimensional conformal radiation therapy (3D-CRT) technique. The fractionation for external beam RT (EBRT) was a 1.8 Gy tumor dose daily with 5 fractions per week to the pelvis, PALN and SCLN regions. The doses to the whole pelvis ranged from 50.4 to 66.6 Gy (median, 55.8 Gy), including the boost dose to the parametrial and/or pelvis lymph nodes (PLNs). Beyond 45-54 Gy to the whole pelvis, shrinking field technique was used in patients who had treated boost RT to the parametrial and PALNs. The range of the PALN dose was 54-63 Gy, with a median of 54 Gy. The gross tumor (including the involved PALNs) and the elective PALN area were treated with a boost to the tumor bed. Most patients were treated with four-fields based on 3D-CRT. The PALNs and pelvis RT field were irradiated with an extended field that includes both the PALNs and the pelvis (Fig. 1). The upper margin of the field was at the T12-L1 interspace and the lower margin was at the ischial tuberosity. The width of the PALN field (generally 8-10 cm) was determined by the PALN involvement. A technique using four isocentric fields equally weighted was used and the RT was delivered through a linear accelerator that produced 10 MV photons. During the time of receiving EBRT, all of the patients were treated with high-dose rate (HDR) brachytherapy. The brachytherapy with HDR was performed with a dose of 21-32 Gy (median, 24 Gy) in two weekly fractions of 3.5-5 Gy (median, 4 Gy) to point A, which was defined by the Manchester system as 2 cm above the vaginal fornix and 2 cm lateral to the midline. The doses to the SCLN ranged from 60 to 75.6 Gy (median, 66.6 Gy) with the RT strategy consisting of conventional RT and/or 3D-CRT. For the SCLN conventional RT, a single anterior treatment field was set at 100 cm source-to-skin distance (SSD) by using an asymmetric inferior field border such that the lower jaw was placed at the central axis, at the base of the head of the clavicle. The gantry was angled 10° away from the spinal cord, and a focused block was placed to maintain an off-cord approach (Fig. 1). Beyond 36-50.4 Gy to the SCLN area, boost RT to gross tumor (the involved SCLNs) using shrinking field technique was performed. All 7 patients were treated with cisplatin-based chemotherapy to RT. All 7 patients received the 5-fluorouracil (5-FU) and cisplatin chemotherapy during RT. The chemotherapy regimens consisted of cisplatin 40-60 mg/m2 by intravenous infusion given on day 1 and 5-FU at a dose of 1,000 mg/m2 per day for 5 days given as a continuous infusion on days 1 to 5. Chemotherapy was repeated every 3 weeks. There was no RT field and dose modification. Chemotherapy was also completed without interruption in all patients. Acute toxicities during CCRT were documented according to the RTOG acute radiation morbidity scoring criteria.
In this study, the response evaluation after CCRT was the primary endpoint. The secondary endpoints were the 3-year and 5-year progression-free survival (PFS) rates (the patterns of locoregional and distant failure) and toxicity. The tumor (pelvis, PALN, and SCLN) response was evaluated with CT scan or PET scan after treatment and was classified as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) according to the WHO response criteria [5]. By use of the Kaplan-Meier method, the OS was calculated from the date of start of RT until the death or last follow-up. PFS was calculated from the date of start of RT until failure (or tumor progression) or latest follow-up date where no recurrence occurred. The patients were censored from the time of last follow-up or death.
The patients' ages ranged from 32 to 69 years with a median age of 48 years. The patients' characteristics and raw data are shown in Tables 1 and 2. All of the patients were staged according to the current FIGO staging system [6].
Based on the radiological and physical examination, a response evaluation for CCRT was performed in all 7 patients. For the primary pelvic tumors, the evaluation of a response was possible in 6 out of 7 patients, since 1 patient received RT after surgery. Therefore it was not possible to evaluate the response after CCRT because there was no primary tumor after surgery. Since there was no primary tumor after surgery to evaluate the response after CCRT. After the completion of CCRT, the evaluation of the PALN response was possible in all 7 patients. Among these 7 patients, 1 patient developed local failure in the PALN after PALN RT. The SCLN response evaluation was also performed in all 7 patients. Table 3 shows the response of 7 patients with SCLN involvement. Fig. 2 is the image showing an example case with multiple PALN and left SCLN metastases.
The median follow-up period was 79 months (range, 13 to 98 months). The 3-year and 5-year OS rates were 57.1% and 57.1%, respectively, and the 3-year and 5-year PFS rates were 57.1% and 42.9%, respectively. The median OS time was 82 months (SD, 70 months; range, 0 to 213 months), and the median PFS time was 37 months (SD, 34 months; range, 0 to 104 months). The OS and PFS curve using the Kaplan-Meir method is shown in Fig. 3.
Of the 7 patients, locoregional and distant failures occurred in 3 (43%) and 2 patients (29%), respectively. Table 3 shows the patterns and sites of failure in 7 patients with SCLN involvement. The mean time to failure was 30.7 months for locoregional disease and 22.5 months for distant failure. Among all patients, 2 patients without any treatment failure were alive and 1 patient with treatment failure (in bone) was also alive.
The acute toxicities according to the RTOG criteria are summarized in Table 4. The most common hematologic toxicity was G3/4 leukopenia in 5 (71%). Within 6 months after RT, most of the patients (4/5, 80%) recovered from the G3/4 leukopenia, except for 1 patient who received chemotherapy after completing RT due to subsequent bone metastasis.
Patients with SCLN involvement at the time of the primary diagnosis generally have poor outcomes. The reported 5-year survival rates of patients with SCLN involvement were 16.5%. That study concluded that primary SCLN metastasis in patients with cervical cancer was not incurable [7]. Tran et al. [8] reported a median survival of 7.5 months in 14 cervix cancer patients with SCLN involvement. In this report, the curative aim treatment was performed in 7 patients. Most patients (5/7, 71%), received pelvis and PALN RT including brachytherapy with concurrent cisplatin chemotherapy. The other 2 patients (2/7, 29%) received SCLN RT. In addition, another study including 12 cervical cancer patients with SCLN involvement reported a 24.7% 2 year OS rate [9].
Our study showed favorable outcomes. There was disagreement in the previous results reported poor results [7-9]. However, Hong et al. [10] reported that the 3-year survival rate was 50% for patients with SCLN with or without PALN relapse treated by CCRT/RT. They also suggested that aggressive CCRT/RT may significantly improve patient survival from the results and concluded that patients with SCLN relapse had a longer survival time than those other metastases (except PALN). Direct comparisons of our study with previous reports were not possible because of difference in patients' characteristics and the study design. Although the response and survival rates in our preliminary study are encouraging, this study has several limitations. First, the number of patients was too small to make a conclusion about the survival outcomes. Nevertheless, these favorable results suggest the importance of aggressive therapy for the selected patients who have cervical cancer with SCLN involvement.
Some of the patients with PALN involvement had micrometastases to the left SLCNs [11]. The rationale of the SCLN RT for cervical cancer patients with SCLN involvement relies on the following two reasons. First, for patients with cervical cancer, the pattern of lymphatic spread appears to be stepwise from the pelvic lymph nodes to the PALNs and finally to the SCLNs [12]. Based on the patterns of lymphatic spread in cervical cancer, we considered cervix cancer with SCLN involvement as a localized disease and postulate that the aggressive therapy including CCRT may improve the outcomes if there is no evidence of tumor spread outside of the SCLN. This is the first reason why we expected that the prognosis of cervical cancer with SCLN involvement has somewhat different significance compared with the other cancers with SCLN involvement. However, accurate evaluation before treatment should be performed to rule out the possibility of hematogenous metastasis to other organs.
Second, from the retrospective results of PALN RT, we expected that the effect of CCRT would be better than that with just chemotherapy alone or with observation for cervical cancer patients with SCLN involvement. We have previously reported that patients with PALN involvement treated by CCRT showed favorable survival outcomes (3-year OS rate 63.6% and 3-year PFS rate 56.4%) with acceptable toxicities [13]. Such favorable outcome is one of the reasons why we further evaluated treatment outcomes of patients with more advanced stages. There have recently been an increasing number of reports showing the favorable results after extended-field RT (PALN RT) for advanced cervical cancer with positive PALNs. PALN RT is generally indicated for patients with both pelvic tumors and PALN involvement. Although the role of SCLN RT has not yet been determined due to the very small number of patients with SCLN involvement and the lack of randomized trials, these PALN RT results provide the foundation for SCLN RT. In a retrospective analysis of 43 patients with stage I, II, or III cervical cancer and biopsy-proven positive PALNs and who had been treated with extended field RT and low-dose-rate brachytherapy, the 5-year OS rate was 32% and the cause-specific survival rate was 49%. The authors reported that the majority of relapses occurred at distant sites [14]. They reported that the most frequently observed grade 3 or greater acute toxicities were hematologic, and these acute toxicities occurred in 23 patients (70%) and they were usually self-limited. This results are similar to our results showing that the most common acute toxicity were also hematologic and most of the patients recovered from the hematologic toxicities within 6 months after RT. These preliminary results indicate that the PALN RT, including supraclavicular fossa (SCF) RT, did not ameliorate the acute toxicities compared with the acute toxicities of the reported PALN RT alone. The main cause of the higher hematologic toxicities are considered to be due to the PALN RT rather than to the SCF RT and the combined chemotherapy. We expected that the toxicities in patients with SCLN involvement treated by CCRT will not be far worse than patients with PALN involvement treated by CCRT, and the review on toxicities and ways of reducing toxicities with patients with PALN involvement would be also important for reducing the toxicities in patients with SCLN involvement. There are several ways to reduce the toxicities related to CCRT. The first way to reduce the toxicities (and especially the hematologic ones) is to treat the patients who are undergoing CCRT with the combined administration of a transfusion and/or colony-stimulating factor (CSF). Most of the reported hematologic toxicities were usually self-limited, yet the administration of a transfusion and/or CSF is needed to help a patient recover from grade 4 hematologic toxicities [15]. The second way of reducing the toxicity is to use advanced RT techniques such as intensity modulated radiation therapy (IMRT). The increased conformality of IMRT can permit dose escalation to tumor that is surrounded by radiosensitive normal tissues. Portelance et al. [16] have shown that treatment of the pelvis and the PALNs with the IMRT technique can significantly spare normal tissue. The University of Alabama has shown that it is possible to escalate the dose to 60 Gy using the IMRT technique [17]. The third way is to develop chemotherapy regimens with fewer side effects. A considerable part of the hematologic toxicities may be related to chemotherapy. There are a large number of chemotherapeutic agents for treating recurrent or stage IVB SCC of the cervix. Among these chemotherapeutic agents, cisplatin is the single most active agent to treat cervical cancer [18]. The cisplatin-based chemotherapy regimens were also performed in our study. In a previous study that used extended field RT with or without chemotherapy, excellent tumor control and substantial acute toxicity were reported when administering a regimen of extended-field radiotherapy with concomitant cisplatin, although it was a preliminary study with limited follow-up [19]. There is a report to reduce the toxicities using hyperfractionated RT (HFRT) techniques [20]. HFRT is a technique of intensifying the RT effect by delivering smaller dose fractions (e.g., 1.2 Gy per fraction) that are given more than once per day, and so this increases the total daily dose to 2.4 Gy or more. Theoretically, this technique has a greater effect against cancer cells and it keeps the chronic toxicities at acceptable levels because of the low dose per fraction. However, there is still controversy regarding its effectiveness in clinical studies [21].
Actually, the number of patients is too small and heterogeneous for prognostic factor analysis in our study. In a previous study that evaluated the outcome and prognostic factors of 33 patients with histologically confirmed SCLN metastasis at the time of the primary diagnosis, multivariate analysis showed that a serum level of SCC-Ag<15 ng/mL at the time of the initial diagnosis (p=0.021) and staging/restaging with including [18F] fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) (p=0.006) was associated with a better prognosis [5].
Although the clinical biology and prognosis of patients with SCLN relapse cannot be compared with patients presenting with SCLN involvement at the time of primary presentation, we further analyzed 2 patients with SCLN relapse salvaged by CCRT. The sites of SCLN involvement of the relapsed 2 patients were on the right and bilateral, respectively. The results showed that one patient (initially, stage IB) out of 2 recurrent patients achieved a complete response after CCRT and long-term survival, shown in Fig. 4.
The RT dose to the SCLN for local control has not been established yet. In our study, we gave higher RT doses to SCLN, because patients had multiple small to medium sized tumors or large single tumor in SCLN. The response rate of SCLN to RT, given 66 Gy or more, was worse (2/4, 50% CR and 2/4, 50% PR) compared to those tumors given less than 66 Gy (3/3, 100% CR). This may be due to selection bias. The multiple or large tumors were given 65 Gy or more, and single or smaller tumor was given less than 66 Gy in principle. The RT dose to SCLN was not an independently significant factor for local control on analysis (data not shown). Similar results were observed for the results of PALN response.
The results of this study shows that the sites of SCLN involvement at the initial presentation were on the left, whereas the sites of SCLN involvement of the relapsed 2 patients were on the right and bilateral, respectively. A previous study that analyzed 219 cervical cancer patients who were treated by RT reported that SCLN metastasis developed in 219 (1.62%): 83.1% on the left, 7.7% on the right and 9.13% bilateral [4]. For the diagnosis of SCLN involvement in our study, sonographically guided fine-needle aspiration biopsy or/and whole body PET was performed for all 7 patients. A previous report that evaluated the frequency and prognostic significance of occult SCLN involvement identified by PET in patients with cervical carcinoma showed that that the positive predictive value of abnormal PET uptake in the left SCLNs was 100%, and the frequency of positive PET uptake in the left SCLNs was 40% (14/35) in those patients with PALN uptake and it was 15% in those patients with stage IIIB disease [8]. For as accurate treatment decision, PET may be helpful in staging the cervical cancer patients with SCLN involvement.
Previous studies that evaluated results of patients after being treated with PALN RT and the corresponding results of the present study are summarized in Table 5.
In conclusion, RT with chemotherapy as active therapy can be expected to provide favorable results for appropriately selected cervical cancer patients with SCLN involvement but no evidence of distant metastasis, although there is an increased risk of G3/4 hematologic toxicity. Although our preliminary results show that the long-term survival, the well designed study enrolling more patients will be necessary to clarify the future indications and selection criteria for CCRT.
Figures and Tables
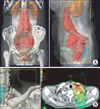 | Fig. 1The three-dimensional conformal radiation therapy fields generated by the treatment-planning computer. (A) The four field plan for the pelvis and para-aortic lymph node radiation therapy (RT). (B) The supraclavicular lymph node conventional RT plan and the image showing the isodose distribution. |
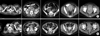 | Fig. 2An example case with multiple para-aortic and left supraclavicular lymph node metastases. A 49-year-old woman who was diagnosed as having uterine cervix cancer with coincidental para-aortic and left supraclavicular lymph node metastases (A, on December 2003) underwent concurrent chemotherapy and radiation therapy with the extended 4 field technique, followed by the supraclavicular one port source-to-skin distance technique. The patient is now on regular follow-up without any evidence of recurrence (B, after treatment on June 2006). |
 | Fig. 3Progression-free survival (PFS) and overall survival (OS) of performing concurrent chemoradiation therapy for cervical cancer with supraclavicular involvement at the initial presentation. |
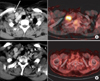 | Fig. 4Example of a case with right supraclavicular lymph node metastasis (A, before treatment). The patient underwent concurrent chemotherapy and radiation therapy by the supraclavicular one port source-to-skin distance technique. On regular follow-up, the right supraclavicular lymph node metastasis disappeared (B, after treatment). |
References
1. Perez CA, Camel HM, Kuske RR, Kao MS, Galakatos A, Hederman MA, et al. Radiation therapy alone in the treatment of carcinoma of the uterine cervix: a 20-year experience. Gynecol Oncol. 1986. 23:127–140.
2. Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of Radiation Therapy Oncology Group trial (RTOG) 90-01. J Clin Oncol. 2004. 22:872–880.
3. Kim K, Cho SY, Kim BJ, Kim MH, Choi SC, Ryu SY. The type of metastasis is a prognostic factor in disseminated cervical cancer. J Gynecol Oncol. 2010. 21:186–190.
4. Yao ZH, Wu AR. Supraclavicular lymph node metastasis from carcinoma of the uterine cervix after radiotherapy - analysis of 219 patients. Zhonghua Zhong Liu Za Zhi. 1988. 10:230–232.
5. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981. 47:207–214.
6. Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol Obstet. 2009. 105:107–108.
7. Qiu JT, Ho KC, Lai CH, Yen TC, Huang YT, Chao A, et al. Supraclavicular lymph node metastases in cervical cancer. Eur J Gynaecol Oncol. 2007. 28:33–38.
8. Tran BN, Grigsby PW, Dehdashti F, Herzog TJ, Siegel BA. Occult supraclavicular lymph node metastasis identified by FDG-PET in patients with carcinoma of the uterine cervix. Gynecol Oncol. 2003. 90:572–576.
9. Chao A, Ho KC, Wang CC, Cheng HH, Lin G, Yen TC, et al. Positron emission tomography in evaluating the feasibility of curative intent in cervical cancer patients with limited distant lymph node metastases. Gynecol Oncol. 2008. 110:172–178.
10. Hong JH, Tsai CS, Lai CH, Chang TC, Wang CC, Chou HH, et al. Recurrent squamous cell carcinoma of cervix after definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2004. 60:249–257.
11. Stehman FB, Bundy BN, Hanjani P, Fowler WC, Abdulhay G, Whitney CW. Biopsy of the scalene fat pad in carcinoma of the cervix uteri metastatic to the periaortic lymph nodes. Surg Gynecol Obstet. 1987. 165:503–506.
12. Beyer FD Jr, Murphy A. Patterns of spread of invasive cancer of the uterine cervix. Cancer. 1965. 18:34–40.
13. Shin JW, Lee KC, Lee SH, Choi JH, Lee KB, Park CY. Concurrent chemoradiation therapy in cervical cancer with para-aortic lymph node involvement. Korean J Gynecol Oncol. 2007. 18:108–113.
14. Grigsby PW, Perez CA, Chao KS, Herzog T, Mutch DG, Rader J. Radiation therapy for carcinoma of the cervix with biopsy-proven positive para-aortic lymph nodes. Int J Radiat Oncol Biol Phys. 2001. 49:733–738.
15. Kim YS, Kim JH, Ahn SD, Lee SW, Shin SS, Nam JH, et al. High-dose extended-field irradiation and high-dose-rate brachytherapy with concurrent chemotherapy for cervical cancer with positive para-aortic lymph nodes. Int J Radiat Oncol Biol Phys. 2009. 74:1522–1528.
16. Portelance L, Chao KS, Grigsby PW, Bennet H, Low D. Intensity-modulated radiation therapy (IMRT) reduces small bowel, rectum, and bladder doses in patients with cervical cancer receiving pelvic and para-aortic irradiation. Int J Radiat Oncol Biol Phys. 2001. 51:261–266.
17. Ahmed RS, Kim RY, Duan J, Meleth S, De Los Santos JF, Fiveash JB, et al. IMRT dose escalation for positive para-aortic lymph nodes in patients with locally advanced cervical cancer while reducing dose to bone marrow and other organs at risk. Int J Radiat Oncol Biol Phys. 2004. 60:505–512.
18. Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL 3rd, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999. 340:1154–1161.
19. Sood BM, Gorla GR, Garg M, Anderson PS, Fields AL, Runowicz CD, et al. Extended-field radiotherapy and high-dose-rate brachytherapy in carcinoma of the uterine cervix: clinical experience with and without concomitant chemotherapy. Cancer. 2003. 97:1781–1788.
20. Kim JS, Kim JS, Kim SY, Kim KH, Cho MJ. Hyperfractionated radiotherapy with concurrent chemotherapy for para-aortic lymph node recurrence in carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2003. 55:1247–1253.
21. Grigsby PW, Lu JD, Mutch DG, Kim RY, Eifel PJ. Twice-daily fractionation of external irradiation with brachytherapy and chemotherapy in carcinoma of the cervix with positive para-aortic lymph nodes: phase II study of the Radiation Therapy Oncology Group 92-10. Int J Radiat Oncol Biol Phys. 1998. 41:817–822.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download