Abstract
Objective
The aim of this study was to investigate the risk and recurrence of early invasive adenocarcinoma of the cervix, and to determine whether non-radical methods of management could be performed.
Methods
The medical and histopathological records of 50 patients with early invasive adenocarcinoma of the cervix treated at Keio University Hospital between 1993 and 2005 were reviewed, and compared with the literature.
Results
The median follow-up period was 64.3 months. The depth of stromal invasion was ≤3 mm in 33 cases and >3 mm, but ≤5 mm in 17 cases. The horizontal spread was ≤7 mm in 25 cases and >7 mm in 25 cases. One of the 33 cases that had tumor volumes of ≤500 mm3, and three of the 17 cases with tumor volumes of >500 mm3 were positive for lymph node metastasis. When our data were combined with previously reported results, statistically significant differences were observed between the tumor volume and the frequency of pelvic lymph node metastasis/the rate of recurrence (p<0.0001). The frequency of pelvic lymph node metastases was significantly higher in the lymphovascular space invasion (LVSI)-positive group than in the LVSI-negative group (p=0.02). No adnexal metastasis or parametrial involvement was noted.
Conclusion
Assessment of the depth of stromal invasion, tumor volume, and LVSI is critical for selecting an appropriate therapeutic modality. Non-radical methods of management are considered suitable for patients with LVSI-negative adenocarcinoma of the cervix exhibiting a stromal invasion depth of ≤5 mm and a tumor volume of ≤500 mm3.
The increasing incidence of adenocarcinoma of the cervix in the world, especially amongst young women, makes discussion of non-radical methods of management for early invasive adenocarcinoma of the cervix highly relevant. However, the optimal treatment for this cancer has not yet been established because of the poor understanding of its natural history, histopathological criteria, and morphology, as well as discordances in measurements of stromal invasion. Therefore, treatment usually consists of radical hysterectomy [1-3]. Recently, studies have reported no differences in survival among properly selected cases of early invasive adenocarcinoma of the cervix managed using radical hysterectomy [4-13] or non-radical methods of management [6,8,14,15]. Therefore, it is necessary to investigate prognostic factors for the application of optimal treatment.
In previous studies on early invasive adenocarcinoma of the cervix, the importance of the depth of stromal invasion was stressed [6,7,16]. Therefore, many studies evaluated FIGO stages IA1 and IA2 adenocarcinoma of the cervix and concluded non-radical methods of management may be considered suitable because of a good prognosis. However, the cases of adenocarcinoma of the cervix with stromal invasion depth of ≤5 mm and horizontal spread of >7 mm are seen occasionally. Therefore, this study included cases with a stromal depth invasion of ≤5 mm unrelated to the horizontal spread and discussed with respect to the suitability of non-radical methods of management and prognostic factors.
The associations between patient outcome and clinical parameters, including histopathological diagnosis, surgical procedure, depth of stromal invasion, horizontal spread, tumor volume, lymphovascular space invasion (LVSI), status of pelvic lymph node metastasis, and status of parametrial tissue invasion were assessed. We investigated the risk of metastasis and recurrence of early invasive adenocarcinoma of the cervix by analyzing clinicopathologic factors among retrospective cases at our institution, and aimed to determine whether these patients were suitable for non-radical methods of management.
We examined the clinical records and histopathological specimens of 50 patients with adenocarcinoma of the cervix with a stromal invasion depth of ≤5 mm who had undergone surgical treatment. All patients were treated at the Department of Obstetrics and Gynecology, Keio University Hospital, Tokyo, Japan, between January 1993 and December 2005. Eight to 12 sections from cervical tissues were obtained for hematoxylin and eosin staining, depending on the size of each uterine cervix. Histology was classified using the criteria of the 2003 World Health Organization international histological classification for tumors of the uterine cervix. Patients with common histological subtypes, mucinous adenocarcinoma of the endocervical type, endometrioid adenocarcinoma, or adenosquamous carcinoma were included in the analysis, while those with rare types, such as clear cell carcinoma, were excluded. We evaluated tumor histology, depth of stromal invasion, horizontal spread of the tumor, tumor volume, presence/absence of LVSI, presence/absence of metastasis to the pelvic lymph nodes or adnexa, presence/absence of parametrial tissue invasion, treatment, and outcome.
We also examined the association between the margin status of conization and the presence of residual tumor in subsequently obtained hysterectomy specimens from 13 cases in whom conization was performed preoperatively. If conization had been performed preoperatively and the surgical margin was positive for tumor cells, the maximum depth of stromal invasion and the horizontal spread were re-estimated using the whole resected specimen obtained by both conization and subsequent hysterectomy.
The depth of stromal invasion was measured from the base of the surface epithelium to the deepest detectable malignant cells using an ocular micrometer [10]. The volumes were estimated from the section with the largest tumor surface area by multiplying three dimensions: depth of stromal invasion, horizontal spread, and the third dimension. The third dimension was calculated using the Burghardt method, as 1.5 times the largest measured depth of stromal invasion or horizontal spread (Fig. 1) [16]. LVSI was defined by the unequivocal presence of malignant cells in endothelium-lined spaces in the hysterectomy specimens or conization specimens. All clinical patient information was obtained from medical records.
The statistical differences between the presence of pelvic lymph node metastasis/the frequency of recurrence and tumor volume were determined using Fisher's exact test, as were the statistical differences between the presence of LVSI and that of pelvic lymph node metastasis. The statistical differences between the presence of pelvic lymph node metastasis and histopathological diagnosis/depth of stromal invasion/horizontal spread/tumor volume were determined using Wilcoxon's signed rank test. Differences with a p-value of <0.05 were considered significant.
The mean age of the patients was 45 years, ranging from 26 to 87 years, and the median follow-up period was 64.3 months, ranging from 8.9 to 162.4 months. Neither recurrences nor deaths were recorded during the follow-up period. The histological subtypes included endocervical mucinous adenocarcinoma in 29 cases, endometrioid adenocarcinoma in seven cases, and adenosquamous carcinoma in 14 cases. All patients had undergone a hysterectomy (radical hysterectomy in 43 cases, and modified radical hysterectomy in seven cases). Only one patient (patient 1 in Table 1) received postoperative adjuvant radiotherapy.
Thirteen cases underwent conization preoperatively. All three cases with positive margins for invasive disease at the time of conization showed residual invasive disease at the time of the subsequent hysterectomy. None of the remaining 10 cases with a negative margin showed residual invasive disease in subsequently obtained surgical specimens.
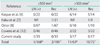
In 33 cases, the depth of stromal invasion was ≤3 mm. In the remaining 17 cases, the depth of stromal invasion was >3 mm but ≤5 mm. The horizontal spread was ≤7 mm in 25 cases, >7 mm but ≤10 mm in 10 cases, and >10 mm in 15 cases. The horizontal spread of all four cases with positive for lymph node metastasis was >7 mm. Statistically significant differences were observed between the frequency of pelvic lymph node metastasis and horizontal spread (p=0.03). The median calculated tumor volume was 270 mm3, ranging from 0.75 mm3 to 27,000 mm3. Thirty-three cases (66%) had tumor volumes of ≤500 mm3, and 17 cases (34%) had tumor volumes of >500 mm3. One of the 33 cases that had tumor volumes of ≤500 mm3, and three of the 17 cases with tumor volumes of >500 mm3 were positive for lymph node metastasis. There was no difference in tumor volume between those positive for lymph node metastasis and those negative for lymph node metastasis (p=0.086). Comparison of the two groups was made by Wilcoxon's signed rank test. Statistically significant differences were not observed in this study, but when our data were combined with previously reported results [6-8,12], statistically significant differences were observed between tumor volume and frequency of pelvic lymph node metastasis (p<0.0001) and between tumor volume and rate of recurrence (p<0.0001), using a tumor volume of ≤500 mm3 as a cut-off (Table 2). LVSI was present in 11 cases, and three of these 11 cases were positive for pelvic lymph node metastasis. The frequency of pelvic lymph node metastasis was significantly higher in the LVSI-positive group than in the LVSI-negative group (p=0.02) (Table 3). In four of these 11 cases, the depth of stromal invasion was ≤3 mm and the horizontal spread ranged from 11 mm to 35 mm. In seven of the 11 cases, the depth of stromal invasion was >3 mm but ≤5 mm, and the horizontal spread ranged from 7 mm to 13 mm. Bilateral adnexa had been removed in 35 cases, and unilateral adnexa had been removed in 11 cases at the time of hysterectomy. None of the cases had metastasis in the resected tissues, and none showed parametrial involvement.
The clinicopathological features of cases of LVSI-negative adenocarcinoma of the cervix with a tumor volume ≤500 mm3 were as follows. Thirty-one cases were LVSI-negative with a tumor volume ≤500 mm3. The median depth of stromal invasion was 3 mm, ranging from 0.5 to 5 mm. The median horizontal spread was 6 mm, ranging from 1 to 12 mm. The median tumor volume was 150 mm3, ranging from 0.75 to 432 mm3. There were no cases that were positive for pelvic lymph node metastasis.
Identification of reliable and reproducible prognosis factors in carcinoma of the cervix, especially stage I, are important for customization of treatment for patients. Thus, certain clinical and pathological risk factors have been investigated. Previous studies have categorized depth of stromal invasion, large tumor diameter, and LVSI as independent risk factors because of their frequent association with increased lymph node metastases, recurrence, and poor survival. Among these three risk factors, depth of stromal invasion is the most critical [25-29]. Recently, several studies evaluated clinicopathologic features and follow-up of early invasive adenocarcinoma of the cervix for determining if the patients could undergo non-radical methods of management. Many studies evaluated FIGO stages IA1 and IA2 adenocarcinoma of the cervix and concluded non-radical methods of management might be considered suitable because of a good prognosis. However, the cases of adenocarcinoma of the cervix with stromal invasion depth of ≤5 mm and horizontal spread of >7 mm are seen occasionally. Thus, we analyzed patients with stromal invasion of ≤5 mm unrelated to the horizontal spread without macroscopic carcinoma for adaptation to non-radical methods of management. The clinicopathological features of cases of adenocarcinoma of the cervix with a stromal invasion depth of ≤5 mm and horizontal spread of >7 mm were as follows. Twenty-five cases had stromal invasion of ≤5 mm and horizontal spread of >7 mm. The median depth of stromal invasion was 3 mm, ranging from 0.5 to 5 mm. The median horizontal spread was 13 mm, ranging from 8 to 60 mm. The median tumor volume was 937 mm3, ranging from 96 to 27,000 mm3. LVSI was present in 10 cases, and four cases were positive for pelvic lymph node metastasis. Though the previous studies reported depth of stromal invasion as the most critical for evaluating lymph node metastasis or recurrence [25-29], we aimed to investigate other prognostic factors of early invasive adenocarcinoma of the cervix, and to determine whether non-radical methods of management could be performed.
Some researchers have reported that tumor volume may be a more reliable indicator for prognosis than depth of stromal invasion, and this parameter has been applied for the evaluation of patients with early invasive adenocarcinoma of the cervix [16]. Many studies have evaluated FIGO stages IA1 and IA2 adenocarcinoma of the cervix. However, only four papers [6-8,12] compared tumor volume of early invasive adenocarcinoma of the cervix and lymph node metastasis or recurrence. Moreover, tumor volume was calculated using the Burghardt method in all four reports: depth×horizontal spread×1.5 the greater value for either depth or horizontal spread. Most of the previous studies used tumor volumes of 500 mm3 as a cut-off for non-radical methods of management [6-8,12]. Using tumor volume rather than depth of stromal invasion as the prognostic criterion, they concluded that conization or a simple hysterectomy may be appropriate in patients with a tumor volume of ≤500 mm3. Statistically significant differences were not observed in this current study, but when our data were combined with previously reported results [6-8,12], statistically significant differences were observed between tumor volume and frequency of pelvic lymph node metastasis (p<0.0001), and between tumor volume and rate of recurrence (p<0.0001), using a tumor volume of ≤500 mm3 as a cut-off (Table 2). These data suggest that the tumor volume may be a highly reliable prognostic factor for early invasive adenocarcinoma of the cervix.
Our data demonstrated a statistically significant difference between LVSI and frequency of pelvic lymph node metastasis (p=0.02) (Table 3). Patients that have LVSI-negative adenocarcinoma of the cervix with a stromal invasion depth of ≤5 mm might not require a pelvic lymph node dissection.
Fujii et al. [30] reported that innovative digital narrow band imaging colposcopy is useful for identifying early invasive adenocarcinoma of the cervix. Despite this, conization has the advantage of providing information regarding the extent of invasiveness, tumor volume, and LVSI. In patients suspected of having early invasive adenocarcinoma of the cervix, diagnostic conization to evaluate tumor volume and LVSI is preferential prior to non-radical methods of management in principle, unless the tumor is a macroscopic invasive adenocarcinoma.
In this study, none of the cases had ovarian metastases and parametrial involvement, supporting the results of previous studies [10,13,14,18-24]. Accordingly, oophorectomy and resection of the parametrium can be avoided in cases with adenocarcinoma of the cervix with a depth of stromal invasion of ≤5 mm.
In summary, assessment of the depth of stromal invasion, tumor volume and LVSI are critical when selecting an appropriate therapeutic modality by diagnostic conization prior to treatment. An extrafascial hysterectomy with excision of the anterior leaf of the vesicouterine ligament, without lymph node dissection and oophorectomy, which we define as 'non-radical methods of management', are acceptable for patients with LVSI-negative cervical adenocarcinoma of the cervix exhibiting a depth of stromal invasion of ≤5 mm and a tumor volume of ≤500 mm3. A prospective study in a cohort of patients with early invasive adenocarcinoma of the cervix focusing on the pathological review and long-term patient follow-up is required to confirm the safety of non-radical methods of management.
Figures and Tables
Fig. 1
Diagram of tumor volume measurement calculated using the Burghardt method. Tumor volume was determined using the following equation: depth (D) of stromal invasion×horizontal (H) spread×1.5 greater value of depth or horizontal spread.
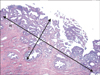
Table 2
Summary of the literature describing tumor volume, pelvic lymph node metastasis and recurrence
References
1. DiSaia PJ, Creasman WT. Clinical gynecologic oncology. 1997. 5th ed. St. Louis: Mosby.
2. Jones WB, Mercer GO, Lewis JL Jr, Rubin SC, Hoskins WJ. Early invasive carcinoma of the cervix. Gynecol Oncol. 1993. 51:26–32.
3. McGonigle KF, Berek JS. Early-stage squamous cell and adenocarcinoma of the cervix. Curr Opin Obstet Gynecol. 1992. 4:109–119.
4. Matsukuma K, Tsukamoto N, Kaku T, Matsumura M, Toki N, Toh N, et al. Early adenocarcinoma of the uterine cervix - its histologic and immunohistologic study. Gynecol Oncol. 1989. 35:38–43.
5. Rollason TP, Cullimore J, Bradgate MG. A suggested columnar cell morphological equivalent of squamous carcinoma in situ with early stromal invasion. Int J Gynecol Pathol. 1989. 8:230–236.
6. Kaspar HG, Dinh TV, Doherty MG, Hannigan EV, Kumar D. Clinical implications of tumor volume measurement in stage I adenocarcinoma of the cervix. Obstet Gynecol. 1993. 81:296–300.
7. Kaku T, Kamura T, Sakai K, Amada S, Kobayashi H, Shigematsu T, et al. Early adenocarcinoma of the uterine cervix. Gynecol Oncol. 1997. 65:281–285.
8. Ostor AG. Early invasive adenocarcinoma of the uterine cervix. Int J Gynecol Pathol. 2000. 19:29–38.
9. Kurian K, al-Nafussi A. Relation of cervical glandular intraepithelial neoplasia to microinvasive and invasive adenocarcinoma of the uterine cervix: a study of 121 cases. J Clin Pathol. 1999. 52:112–117.
10. Nicklin JL, Perrin LC, Crandon AJ, Ward BG. Microinvasive adenocarcinoma of the cervix. Aust N Z J Obstet Gynaecol. 1999. 39:411–413.
11. Elliott P, Coppleson M, Russell P, Liouros P, Carter J, MacLeod C, et al. Early invasive (FIGO stage IA) carcinoma of the cervix: a clinico-pathologic study of 476 cases. Int J Gynecol Cancer. 2000. 10:42–52.
12. Covens A, Kirby J, Shaw P, Chapman W, Franseen E. Prognostic factors for relapse and pelvic lymph node metastases in early stage I adenocarcinoma of the cervix. Gynecol Oncol. 1999. 74:423–427.
13. Webb JC, Key CR, Qualls CR, Smith HO. Population-based study of microinvasive adenocarcinoma of the uterine cervix. Obstet Gynecol. 2001. 97(5 Pt 1):701–706.
14. Schorge JO, Lee KR, Flynn CE, Goodman A, Sheets EE. Stage IA1 cervical adenocarcinoma: definition and treatment. Obstet Gynecol. 1999. 93:219–222.
15. Schorge JO, Lee KR, Sheets EE. Prospective management of stage IA(1) cervical adenocarcinoma by conization alone to preserve fertility: a preliminary report. Gynecol Oncol. 2000. 78:217–220.
16. Burghardt E. Microinvasive carcinoma in gynaecological pathology. Clin Obstet Gynaecol. 1984. 11:239–257.
17. Kasamatsu T, Okada S, Tsuda H, Shiromizu K, Yamada T, Tsunematsu R, et al. Early invasive adenocarcinoma of the uterine cervix: criteria for nonradical surgical treatment. Gynecol Oncol. 2002. 85:327–332.
18. Bisseling KC, Bekkers RL, Rome RM, Quinn MA. Treatment of microinvasive adenocarcinoma of the uterine cervix: a retrospective study and review of the literature. Gynecol Oncol. 2007. 107:424–430.
19. Nagarsheth NP, Maxwell GL, Bentley RC, Rodriguez G. Bilateral pelvic lymph node metastases in a case of FIGO stage IA(1) adenocarcinoma of the cervix. Gynecol Oncol. 2000. 77:467–470.
20. Utsugi K, Shimizu Y, Akiyama F, Hasumi K. Is the invasion depth in millimeters valid to determine the prognosis of early invasive cervical adenocarcinoma? A case of recurrent FIGO stage IA1 cervical adenocarcinoma. Gynecol Oncol. 2001. 82:205–207.
21. Smith HO, Qualls CR, Romero AA, Webb JC, Dorin MH, Padilla LA, et al. Is there a difference in survival for IA1 and IA2 adenocarcinoma of the uterine cervix? Gynecol Oncol. 2002. 85:229–241.
22. Hirai Y, Takeshima N, Tate S, Akiyama F, Furuta R, Hasumi K. Early invasive cervical adenocarcinoma: its potential for nodal metastasis or recurrence. BJOG. 2003. 110:241–246.
23. Balega J, Michael H, Hurteau J, Moore DH, Santiesteban J, Sutton GP, et al. The risk of nodal metastasis in early adenocarcinoma of the uterine cervix. Int J Gynecol Cancer. 2004. 14:104–109.
24. Ceballos KM, Shaw D, Daya D. Microinvasive cervical adenocarcinoma (FIGO stage 1A tumors): results of surgical staging and outcome analysis. Am J Surg Pathol. 2006. 30:370–374.
25. Chung CK, Nahhas WA, Stryker JA, Curry SL, Abt AB, Mortel R. Analysis of factors contributing to treatment failures in stages IB and IIA carcinoma of the cervix. Am J Obstet Gynecol. 1980. 138:550–556.
26. Boyce J, Fruchter RG, Nicastri AD, Ambiavagar PC, Reinis MS, Nelson JH Jr. Prognostic factors in stage I Carcinoma of the cervix. Gynecol Oncol. 1981. 12(2 Pt 1):154–165.
27. van Nagell JR Jr, Donaldson ES, Wood EG, Parker JC Jr. The significance of vascular invasion and lymphocytic infiltration in invasive cervical cancer. Cancer. 1978. 41:228–234.
28. Abdulhayoglu G, Rich WM, Reynolds J, DiSaia PJ. Selective radiation therapy in stage IB uterine cervical carcinoma following radical pelvic surgery. Gynecol Oncol. 1980. 10:84–92.
29. Boyce JG, Fruchter RG, Nicastri AD, DeRegt RH, Ambiavagar PC, Reinis M, et al. Vascular invasion in stage I carcinoma of the cervix. Cancer. 1984. 53:1175–1180.
30. Fujii T, Nakamura M, Kameyama K, Saito M, Nishio H, Ohno A, et al. Digital colposcopy for the diagnosis of cervical adenocarcinoma using a narrow band imaging system. Int J Gynecol Cancer. 2010. 20:605–610.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download