Abstract
The most common form of malignant transformation developing from a mature cystic teratoma is squamous cell carcinoma, representing 80% of malignant transformations, while adenocarcinoma accounts for approximately 5%. Because of this rarity, few reports exist of preoperative diagnosis of this tumor by magnetic resonance imaging, in particular with fat suppression techniques. Here, we report magnetic resonance imaging findings and clinical features of a 79-year-old woman with mucinous adenocarcinoma arising from a mature cystic teratoma (measuring 5×6 cm), classified as surgical stage IA. Because of the poor prognosis of malignant transformation, when mature cystic teratomas are detected (even smaller than 5 cm tumor size) in postmenopausal women, serum tumor marker carcinoembryonic antigen levels and fat-suppressed magnetic resonance imaging may be potential indicators of malignant transformation.
Malignant transformation of a mature cystic teratoma is an uncommon complication occurring in approximately 1-3% of all mature cystic teratomas [1,2]. Although any of the constituent tissues of a teratoma has the potential to undergo malignant transformation, squamous cell carcinoma (SCC) is the most commonly associated cancer. Other tumors arising in a mature cystic teratoma include adenocarcinoma, thyroid carcinoma, malignant melanoma, transitional cell carcinoma, sarcoma, carcinoid tumor, and neuroectodermal tumor [1,2].
Malignant change is rarely recognized preoperatively, and no specific symptoms exist that would lead one to suspect malignant transformation developing from a mature cystic teratoma. Because of its rarity, few reports exist of preoperative diagnosis of this tumor by magnetic resonance imaging (MRI), in particular fat-suppressed MRI. We report MRI findings from a very rare case with a malignant transformation comprising of an adenocarcinoma in a mature cystic teratoma, classified as clinical stage IA, and we briefly review the literature on early diagnosis.
A 79-year-old multiparous woman (gravida 2, para 2) was referred to our hospital from a local hospital with a pelvic mass detected by ultrasonography. In the initial pelvic examination, an approximately fist-sized, hard, solid mass was detected in the pelvic cavity. Transvaginal ultrasonography revealed a 5×6 cm sized solid mass with cystic portions on the left ovary. The tumor marker levels was as follows; carcinoembryonic antigen (CEA), 134.7 ng/mL (reference range <5.0 ng/mL); CA-125, 4.6 IU/mL (reference range <25 IU/mL); CA 19-9, 4.8 IU/mL (reference range <37.0 IU/mL). Routine laboratory investigations revealed no abnormalities. Pelvic MRI showed a 6×6 cm sized multiseptated solid-cystic mass with internal calcification and fatty component in the left ovary (Fig. 1A-C). The tumor had an obvious solid component that was relatively large and tended to be more solid. The uterus and the right ovary were unremarkable. Gastroduodenoscopy and colonoscopy were normal. The endometrial biopsy showed negative results.
Exploratory operation was planned with the provisional diagnosis of a malignant transformation of a mature cystic teratoma, based on the age of patient, high level of serum CEA, and MRI findings of a large solid component. In the laparotomy, clear yellowish ascites of about 30 mL were found in the pelvic cavity. Cytologic examination of the ascites was negative. A smooth-surfaced spherical, semisolid cystic tumor measuring 5×6×6 cm was found to originate from the left ovary. Neither rupture of the wall nor capsule involvement was observed. The uterus and right ovary were macroscopically normal. The left ovarian tumor contained mixed yellow sebaceous materials with hair tufts, and a solid, but necrotic, portion measuring 3×4×3 cm was found within the tumor. The frozen biopsy in the operation room, which is a routine procedure for ovarian teratomas in our hospital, disclosed an adenocarcinoma combined with benign cystic teratoma. A simple total hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and pelvic lymphadenectomy were performed.
The pathologic diagnosis was mucinous adenocarcinoma arising from a mature cystic teratoma of the left ovary. The cystic portion of the ovary consisted of well differentiated mucinous adenocarcinoma, which was initially associated with dermoid tissue mature cystic teratoma (Fig. 1C). The uterus and right ovary were normal, and metastases to the omentum and lymph nodes were not observed. From these findings, the case was classified as surgical stage IA. One month after the operation, CEA level returned to normal levels (2.6 ng/mL). Her postoperative course was not eventful.
The most common sign of mature cystic teratoma with malignant transformation may be a pelvic mass without any specific symptoms. However, some clinical features of this event have been reported. First, this tumor is age related; although the ages of patients with this tumor ranged from 21 to 87 years in the literature, this tumor occurs most frequently in postmenopausal women [3-5]. Second, tumor size is an important factor contributing to a differential diagnosis between a malignant and mature cystic teratoma [3-5]. In report by Kikkawa et al. [3], a SCC developing from a mature cystic teratoma was significantly larger than a mature cystic teratoma, the mean size of 37 squamous cell carcinomas developing from mature cystic teratomas was 152.3 mm, and the cutoff size between benign and malignant tumors was 99 mm. On the other hand, the size of adenocarcinoma tumors varied from the smallest tumor size of 4 mm in diameter to 36 mm [6]. These results reveal that tumor size is ineffective in contributing to the preoperative diagnosis of adenocarcinomas arising from mature cystic teratomas.
Measurement of serum tumor markers and imaging are two important tools in the differential diagnosis between benign and malignant ovarian tumors. Our patient had a raised preoperative serum CEA level, but not CA-125 and SCC, similar to the findings in the previously reported case [7]. CA 19-9 is known to be a good marker for mature cystic teratomas, and SCC and CEA have been reported as more useful than CA 19-9 or CA-125 in the diagnosis of malignant transformation of mature cystic teratoma. CEA may be the most useful and SCC was second in determining a differential diagnosis between a mature cystic teratoma and a SCC developing from a mature cystic teratoma [3,7].
The mature cystic teratoma is a tumor that can be easily diagnosed by imaging modalities such as plain radiography, CT and MRI [4,8]. Most mature cystic teratomas show radiolucent shadows on plain radiography and significantly low density in CT because fat is usually contained within the tumor. With MRI, mature cystic teratomas are seen with high signal intensities in both T1-weighed and T2-weighted images, and the presence of fat fluid levels or chemical shift artifacts are also useful findings in the diagnosis of a mature cystic teratoma (Fig. 1A, B). However, few reports exist regarding diagnosis of malignant transformations developing from a mature cystic teratoma with these imaging modalities. Kido et al. [2] reported the MRI findings for six mature cystic teratomas with malignant transformations. They observed solid portions in five of six tumors in MRI, and solid portions in two tumors were enhanced by gadolinium to varying degrees [2]. The presence of solid, friable, or variegated portions within the mature cystic teratoma is an important feature in the diagnosis of malignant transformation, and this tumor generally spreads by direct invasion and peritoneal implantation rather than by metastasis to the regional lymph nodes. In the present case (Fig. 1C), the diagnosis of mature cystic teratoma was easily made through unique MRI findings with fat suppression techniques arising from the fat present within the tumor. The usefulness of MRI with fat suppression techniques has also been reported in the diagnosis of mature cystic teratomas [9,10]. To our knowledge, no other reports of MRI with fat suppression techniques exist in the diagnosis of malignant transformation developing from a mature cystic teratoma. The gross appearance of mature cystic teratoma with malignant transformation has been reported to resemble that of cystic teratomas but tends to be more solid [2,11]. The present case also had an obvious solid component and was relatively large.
We present an unusual case of mucinous adenocarcinoma arising from a mature cystic teratoma with high level of CEA as the only clinical evidence. Fat suppressed MRI may be helpful in the diagnosis of mature cystic teratomas and its' malignant transformation tend to have a relatively larger solid component. Considering that malignant transformation is usually observed in postmenopausal women, the mere presence of a relatively large solid protuberance in such a patient should raise suspicions that malignant transformation may have occurred. Serum tumor marker CEA level and fat-suppressed MRI may be helpful in evaluating whether mature cystic teratomas, even smaller than tumor size <5 cm, have undergone malignant transformation.
Figures and Tables
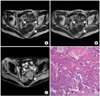 | Fig. 1Mucinous adenocarcinoma arising from a mature cystic teratoma. Magnetic resonance imaging of the pelvis (A-C): Transaxial T1-weighted (A) and T2-weighted images (B), and transaxial T1-weighted image with fat-suppression (C) show a 6×6 cm sized solid-cystic mass with fatty component (white arrows) in the left ovary. Underlying cyst wall is smooth, with no sign of transcapsular extension. Pathohistology (D): The cystic portion of the ovarian tumor consists of well differentiated mucinous adenocarcinoma, which is intimately associated with mature cystic teratoma (H&E, ×200). |
References
1. Griffiths D, Wass J, Look K, Sutton G. Malignant degeneration of a mature cystic teratoma five decades after discovery. Gynecol Oncol. 1995. 59:427–429.
2. Kido A, Togashi K, Konishi I, Kataoka ML, Koyama T, Ueda H, et al. Dermoid cysts of the ovary with malignant transformation: MR appearance. AJR Am J Roentgenol. 1999. 172:445–449.
3. Kikkawa F, Nawa A, Tamakoshi K, Ishikawa H, Kuzuya K, Suganuma N, et al. Diagnosis of squamous cell carcinoma arising from mature cystic teratoma of the ovary. Cancer. 1998. 82:2249–2255.
4. Takemori M, Nishimura R. MRI findings of an ovarian dermoid cyst with malignant transformation. Magn Reson Med Sci. 2003. 2:105–108.
5. Tseng CJ, Chou HH, Huang KG, Chang TC, Liang CC, Lai CH, et al. Squamous cell carcinoma arising in mature cystic teratoma of the ovary. Gynecol Oncol. 1996. 63:364–370.
6. Kushima M. Adenocarcinoma arising from mature cystic teratoma of the ovary. Pathol Int. 2004. 54:139–143.
7. Stewart CJ, Tsukamoto T, Cooke B, Leung YC, Hammond IG. Ovarian mucinous tumour arising in mature cystic teratoma and associated with pseudomyxoma peritonei: report of two cases and comparison with ovarian involvement by low-grade appendiceal mucinous tumour. Pathology. 2006. 38:534–538.
8. Togashi K, Nishimura K, Itoh K, Fujisawa I, Sago T, Minami S, et al. Ovarian cystic teratomas: MR imaging. Radiology. 1987. 162:669–673.
9. Guinet C, Buy JN, Ghossain MA, Malbec L, Hugol D, Truc JB, et al. Fat suppression techniques in MR imaging of mature ovarian teratomas: comparison with CT. Eur J Radiol. 1993. 17:117–121.
10. Imaoka I, Sugimura K, Okizuka H, Iwanari O, Kitao M, Ishida T. Ovarian cystic teratomas: value of chemical fat saturation magnetic resonance imaging. Br J Radiol. 1993. 66:994–997.
11. Yamanaka Y, Tateiwa Y, Miyamoto H, Umemoto Y, Takeuchi Y, Katayama K, et al. Preoperative diagnosis of malignant transformation in mature cystic teratoma of the ovary. Eur J Gynaecol Oncol. 2005. 26:391–392.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download