Abstract
Objective
Photodynamic therapy (PDT) has been used for superficial neoplasms and its usage has been recently extended to deeper lesions. The purpose of this study was to observe whether or not PDT can cure breast cancer in the solid tumor model, and to define the critical point of laser amount for killing the cancer cells.
Methods
Twenty four BALB/c mouse models with subcutaneous EMT6 mammary carcinomas were prepared. Mice were divided into eight groups depending on the amount of illumination, and the tumor size was between 8 mm and 10 mm. We began by peritoneal infiltration with a photosensitizer 48 hours prior to applying the laser light, and then we applied a non-thermal laser light. The energy was from 350 J/cm2 to 30 J/cm2 to the cancer.
Results
Regardless of the tumor size from 8 mm to 10 mm, all mice apparently showed positive results via PDT. We also did not find any recurrence over 90 J/cm2. In all models, the color of the breast cancer lesions began to vary to dark on 2 days post PDT and the tumor regression began simultaneously. Also, we confirmed the complete regression of the breast cancer 21 days after PDT.
Conclusion
We confirmed that PDT may treat breast cancers that are sized less 10 mm in mouse models. The moderate energy to destruct the breast cancer cells may be 90 J/cm2. Therefore, we can expcect that PDT may be utilized to treat breast cancer, but we need more experience, skills and processing for clinical trials.
There were records that ancient civilizations in Egypt, India, China, and Greek employed sun exposure to treat skin diseases, rickets and even psychosis. However, it has not been employed widely in medicine until the eighteen century [1]. In 1903, The group of Von Tappeiner found favorable result after photodynamic therapy (PDT) using several dyes: eosin, fluorescein, sodium dichloroanthracene disulfonate and 'Grubler' s Magdalene red' with light on skin diseases, such as lupus of the skin and condylomata of the female genitalia [2]. Von Tappeiner and Jodlbauer [3,4] proved the necessity of oxygen in this phenomenon in 1904 and in 1907, they explained this phenomenon with oxygen-dependent photosensitization and adopted a term "photodynamic therapy". Although these showed good results, no further reports were forthcoming because of the emergence of ionizing radiation in cancer therapy. Diamond et al. [5] reported in 1972 that hematoporphyrin activated by white light may lead to regression of an experimental glioma in rats. Finally, Dougherty et al. [6] initiated clinical trials of PDT in 25 patients with cutaneous and subcutaneous tumors and confirmed the effectiveness of PDT. After the 1990s, PDT has rapidly become a relevant method both in clinical application and basic mechanistic understanding in treating the skin and superficial cancerous lesions. Now PDT with Photofrin has been approved by the US Food and Drug Administration (FDA) and numerous other health agencies throughout the world for obstructive esophageal cancer and lung cancer, bladder cancer, etc [7]. Recently PDT has been clinically applicable in superficial lesions like skin lesions, cervical intraepithelial lesions and hollowed organs. In our gynecologic department, we have much cumulative data of the effect of PDT on cervical intraepithelial neoplasia and have started to treat breast cancer. However, there are to date no data of the effect of PDT in the field of the breast. So we hereby examined the response of breast cancers to PDT with Photofrin using murine models.
Breast cancer cells (EMT-6) were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and maintained in Waymouth's media supplemented with 15% fetal bovine serum and 1% penicillin-streptomycin solution. 6-week-old female BALB/c mice (Samtako, Osan, Korea) were anesthetized with ketamine and 1.5×105 cells were subcutaneously injected into the lower back. For each experiment, 3 mice were used in each experimental group and treatment was started when the average tumor size was approximately 250 mm3. The study was approved by the local animal welfare committee.
We began by peritoneal infiltration of photosensitizer (Photofrin [HpD], Axcan Pharma Inc., Mont-Saint-Hilaire, Canada) with 5 mg/kg, 48 hours later, we applied a non-thermal laser light (Ceralas Diode Laser 632 System, Biolitec, Jena, Germany) (Fig. 1). The amount of energy was from 350 J/cm2 to 30 J/cm2 on the cancer cell and the margin of laser was a little bit wider than the cancer lesion because cancer can recur easily. Then we evaluated the effects of this procedure every 3 days. The control group was not needed because if the breast cancer was not irradiated, the growth of cancer will certainly continue.
Just after PDT, gray discoloration was shown at the illumination site. The color of illumination site including breast cancer began to darken 2 days post PDT and the cancer regressed simultaneously. The tumor size was from 8 mm to 10 mm, however, regardless of the tumor size all cancer models showed effectiveness of the PDT. However, tissue deformities were noticed in the 240 and 350 J/cm2 group (Fig. 2). We confirmed the complete remission 21 days post PDT from 180 to 90 J/cm2 (Fig. 3).
From 350 J/cm2 to 90 J/cm2, all mice models showed complete remission. However one out of three mice showed no response in the 60 J/cm2 group (Fig. 4) and in the 30 J/cm2 group, the effectiveness of PDT did not show in all mice (Fig. 5). So, we determined that the moderate energy required to treat breast cancer sized less than 10 mm is around 90 J/cm2. We did not find any recurrence in the group that received energy more than 90 J/cm2. So, the energy of 90 J/cm2 may be minimum energy required to destruct the breast cancer cells completely.
PDT is a treatment that can eradicate premalignant and early-staged cancer and can reduce the tumor size in end-staged cancers. The advantage of PDT is that it is a minimally invasive method that can affect the selective area and there is no limitation of repetitions and little complications. It is also has a merit in that PDT is applicable when systemic chemotherapy and surgery are not possible [8]. PDT mechanism can be explained by two parts: uptake of sensitizers and tumor destruction by laser light.
The first approval of PDT was in Canada in 1993 for the treatment of bladder cancers. Esophageal cancer was approved by the US FDA in December 1995, and early non-small cell lung cancer in 1998 followed by Barrett's esophagus [9,10]. Even though PDT data in gynecology are not sufficient, several institutes already have collected much data and some gynecologic departments have tried to begin PDT. Several photosensitizers that can be accumulated in the tumor tissue selectively have been introduced, but the exact mechanisms of each photosensitizers are different. Porphyrin derivatives can be localized in tumors when injected into the bloodstream [11]. In the hematoporphyrin derivative and its improved version, called Photofrin, are still the most widely used photosensitizers in PDT of tumors [12]. Among photosensitizers, Photofrin has been used in our department for the last ten years and we assumed that PDT with Photofrin may be have effective in breast cancers also. In our department, we began to operate on breast cancer from 2010 and we tried to adopt PDT in the breast lesions. So, we conducted this study. We applied the same protocol that was used in patients to the mice model. The drug is injected intravenously and after 48 hours, application of irradiation to tumors was performed with 630 nm wave length of laser [13,14].
PDT has been applicable in the field so that physicians can directly approach the lesion. The breast area is also able to be approach easily, and we can adopt PDT for the breast. The first line treatment for breast cancer is surgery and can lead to changes in the breast shape. Thus, if there is a non-invasive procedure that is able to destroy cancer cells, it would be preferable. Even though there were no reports about PDT for the breast cancer as the first line therapy, several studies showed reliable effects of PDT in locally recurrent breast cancer or chest wall recurrence [15-17]. Thus, we may assume through this data that breast cancer cells may be eradicated by PDT. Also, PDT is applicable during surgery. After mastectomy, PDT can be applied on the bed of the previous lesion for preventing recurrence. The usual laser fiber can be used when PDT is applied to the skin area such as chest wall recurrence. But if we try to treat the lesion that is located under the skin, a specific laser fiber is needed such as an interstitial type that can penetrate the skin and reach the lesion. So, research about the specific laser fiber is mandatory to destroy lesions under the skin.
From our study, first we can reconfirm the PDT effects to destroy breast cancer cells. Next, discovery of proper energy is very important because the amount of energy should be different for each tissue and organ (for example, we applied 240 J/cm2 to uterine cervical cancer). In addition, when photochemical internalization (PCI) concept is added to traditional PDT, the result of cancer cell destruction can be maximized. For performing PCI, chemotherapeutic agents should be added before PDT. When previously infiltrated sensitizers are exposed to light, sensitizers begin to activate and produce free radicals. Finally, endocytosed molecules or chemotherapeutic agents may be released and attack cancer cells directly before being degraded by lysosomes [18]. Thus, if we add this PCI concept with the traditional PDT in our next trial, we can assume that the results will improve markedly.
In our research we were able to confirm the effect of PDT on breast cancer models and found out the proper amount of energy of 90 J/cm2 in less than 10 mm sized breast cancers. However, we assume that we need more technical practice and clinical data to be able to apply PDT to humans.
Figures and Tables
Fig. 1
Photodynamic therapy illumination onto the cancer with a non-thermal laser light (Ceralas Diode Laser 932 System).
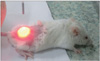
Fig. 2
Complete remission was shown in 350 and 240 J/cm2 but tissue deformities were noticed because of high energy (21 days after photodynamic therapy).

Fig. 3
Complete remission shown in 180, 150, 120, and 90 J/cm2 (21 days after photodynamic therapy).

References
1. Epstein JH. Phototherapy and photochemotherapy. N Engl J Med. 1990. 322:1149–1151.
2. von Tappeiner H, Jesionek A. Therapeutische versuche mit fluoreszierenden stoffen. Munch Med Wochenschr. 1903. (47):2042–2044.
3. von Tappeiner H, Jodlbauer A. Uber die wirkung der photodynamischen (fluorescierenden) stoffe auf protozoen und enzyme. Dtsch Arch Klin Med. 1904. 80:427–487.
4. von Tappeiner H, Jodlbauer A. Die sensibilisierende wirkung fluoreszierender substanzer: gesamte Untersuchungen ber die photodynamische erscheinung. 1907. Leipzig: FCW Vogel.
5. Diamond I, Granelli SG, McDonagh AF, Nielsen S, Wilson CB, Jaenicke R. Photodynamic therapy of malignant tumours. Lancet. 1972. 2:1175–1177.
6. Dougherty TJ, Kaufman JE, Goldfarb A, Weishaupt KR, Boyle D, Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978. 38:2628–2635.
7. Dougherty TJ. An update on photodynamic therapy applications. J Clin Laser Med Surg. 2002. 20:3–7.
8. Hopper C. Photodynamic therapy: a clinical reality in the treatment of cancer. Lancet Oncol. 2000. 1:212–219.
9. Information for patients: lung cancer [Internet]. Oregon Medical Laser Center. c1999. cited 2011 Dec 10. Portland, OR: Oregon Medical Laser Center;Available from: http://omlc.ogi.edu/pdt/ptinfo/lungca/index.html.
10. Photodynamic therapy for cancer [Internet]. National Cancer Institute. c2011. cited 2011 Dec 10. Bethesda, MD: National Cancer Institute;Available from: http://www.cancer.gov/cancertopics/factsheet/Therapy/photodynamic.
11. Hamblin MR, Newman EL. On the mechanism of the tumour-localising effect in photodynamic therapy. J Photochem Photobiol B. 1994. 23:3–8.
12. Juzeniene A, Peng Q, Moan J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem Photobiol Sci. 2007. 6:1234–1245.
13. Photofrin [Internet]. Pinnacle Biologics. c2011. cited 2011 Dec 10. Bannockburn, IL: Pinnacle Biologics;Available from: http://www.photofrin.com.
14. Usuda J, Kato H, Okunaka T, Furukawa K, Tsutsui H, Yamada K, et al. Photodynamic therapy (PDT) for lung cancers. J Thorac Oncol. 2006. 1:489–493.
15. Schuh M, Nseyo UO, Potter WR, Dao TL, Dougherty TJ. Photodynamic therapy for palliation of locally recurrent breast carcinoma. J Clin Oncol. 1987. 5:1766–1770.
16. Sperduto PW, DeLaney TF, Thomas G, Smith P, Dachowski LJ, Russo A, et al. Photodynamic therapy for chest wall recurrence in breast cancer. Int J Radiat Oncol Biol Phys. 1991. 21:441–446.
17. Wyss P, Schwarz V, Dobler-Girdziunaite D, Hornung R, Walt H, Degen A, et al. Photodynamic therapy of locoregional breast cancer recurrences using a chlorin-type photosensitizer. Int J Cancer. 2001. 93:720–724.
18. Hogset A, Prasmickaite L, Selbo PK, Hellum M, Engesaeter BO, Bonsted A, et al. Photochemical internalisation in drug and gene delivery. Adv Drug Deliv Rev. 2004. 56:95–115.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download