Abstract
Objective
We conducted a pilot study to evaluate the effects of pelvic radiotherapy on biologic markers of oxidative stress and plasma endotoxin levels, and to assess the relationship between the changes of such factors and radiotherapy-related complications.
Methods
Twelve gynecologic cancer patients who were treated via pelvic radiotherapy with or without concurrent chemotherapy were enrolled in this study. Biologic markers of oxidative stress, such as glutathione (GSH) and oxidized glutathione (GSSG), as well as endotoxin levels, were measured weekly during treatment. Subjective symptoms were assessed using the Korean version of the EORTC QLQ-C30 at the baseline and on the 5th week of radiotherapy.
Results
No changes were noted in the level of GSH in whole blood, but the GSH/GSSG ratio was reduced dramatically after the initiation of radiotherapy. The mean plasma endotoxin for all patients tended to increase and persisted during radiotherapy, and the number of patients who evidenced clinically significant endotoxin levels (defined as >0.005 EU/mL) also increased. Nausea/vomiting and diarrhea were significantly changed (p=0.019 and p<0.001, respectively). A significant relationship was noted to exist between the changes in the endotoxin level and nausea/vomiting (p=0.001). However, such symptoms did not correlate with the changes of oxidative stress markers.
Conclusion
Pelvic radiotherapy oxidized the GSH redox system and increased plasma endotoxin. Further investigations containing interventional and longitudinal studies will be required to assess the effects of the changes in oxidative stress markers and endotoxin on radiotherapy-related adverse events.
Pelvic radiotherapy is frequently indicated in the treatment of patients with gynecologic cancers [1,2]. However, pelvic radiotherapy can induce a variety of complications, including nausea, vomiting, anorexia, and diarrhea, and ultimately decreases patients' quality of life (QoL) [3-5]. In addition to increasing life expectancy in cancer patients, interventional strategies for minimizing adverse side effects and improving patients' QoL should be considered an urgent necessity. To achieve these goals, investigations should be focused on assessing the role of certain specific factors that might influence such symptoms.
There are some possible biologic markers, the levels of which might be changeable during pelvic radiotherapy. First, as the main therapeutic mechanism of radiation is oxidative damage to cancer cells, it can be assumed that some changes occur in the levels of oxidative stress markers during radiotherapy, resulting in the upregulation of inflammatory cytokines, additional signaling, and the amplification of inflammatory pathways. Endotoxin is another possible factor that might be correlated closely with intestinal toxicities. Endotoxin can enter the systemic circulation due to intestinal barrier break-down by pelvic radiotherapy, and the high incidence of endotoxin in the blood may be associated with nausea, vomiting, abdominal pain, anorexia, and diarrhea, the symptoms most frequently induced by pelvic radiotherapy [6,7].
Although some markers of oxidative stress and endotoxins have been under investigation for the evaluation of their relationship with various diseases, limited information is available regarding the changes in the levels of oxidative stress markers and endotoxins during pelvic radiotherapy, and their correlations with radiotherapy-related adverse events [8-10].
The principal objective of this study was to determine the influence of pelvic radiotherapy with or without concurrent chemotherapy on oxidative stress and plasma endotoxins, and to assess their correlation with radiotherapy-related adverse events.
We conducted a prospective, single-center pilot study at Seoul National University Hospital. A total of 12 patients with endometrial (n=4) or cervical (n=8) cancer voluntarily enrolled in this pilot study between October 2010 and March 2011; the study was approved and overseen by the Institutional Review Board of Seoul National University Hospital in Korea, and all patients provided written informed consent before participating in the study. Characteristics of patients, including their treatments are summarized in Table 1.
All patients underwent radical hysterectomy with pelvic lymph node dissection, and adjuvant radiotherapy with or without chemotherapy was delivered within 2-4 weeks of surgery. Adjuvant radiotherapy was delivered to the whole pelvis at 1.8 Gy per fraction once daily, 5 days per week. The median dose to the whole pelvis was 50.4 Gy. The details of the radiation technique were described in a previous publication [11]. Among 12 patients, nine received adjuvant chemotherapy with radiotherapy. Overnight-fasted venous blood at baseline, 1st, 2nd, 3rd, 4th and 5th weeks of radiotherapy were collected in heparinized tubes and immediately processed.
The total GSH (reduced and oxidized) and GSSG levels were determined using a Glutathione Assay kit (NWK-GSH01, North-west Life Science Specialties, Vancouver, WA, Canada) based on the kinetics of the GSH recycling pathway by GSH reductase [12]. The heparinized whole blood was mixed with 5% metaphosphoric acid (Sigma, St, Louis, MO, USA), vortexed, and centrifuged for 10 minutes at 10,000×g. The supernatants were collected and assayed in accordance with the manufacturer's instructions. In the case of GSSG determination, deproteinized supernatants were further incubated with 4-vivinylpyridine, a thiol-blocking agent, for 60 min at room temperature in order to scavenge free GSH in the sample. Each of the supernatants was mixed with 5-5'-dithiobis (2-nitrobenzoic acid; DTNB), -nicotinamide adenine dinucleotide phosphate (NADPH) and GSH reductase. Samples were incubated in darkness for 3 minutes and then the total GSH was quantified after kinetic spectrophotometric analysis at 412 nm using a Spectra Max 180 (Molecular Devices, Sunnyvale, CA, USA). GSH and GSSG concentrations were calculated from the appropriate calibration curves. Free GSH was obtained by subtracting the concentration of GSSG from total GSH.
Endotoxins in plasma was measured using a Limulus amoebocyte lysate (LAL) assay kit (Diatech, Seoul, Korea) using the kinetic turbidimetric method. We took a great deal of care to prevent possible contamination with exogenous endotoxins or its adsorption to the test materials. Diluted plasma sample with BD100 buffer was heat-inactivated for 10 minutes at 75℃ and then cooled on ice. Samples and appropriate standards from known concentrations of LAL reagent were incubated for 1 hour at 37℃. OD at 395 nm was continuously read at 30 second intervals with an Endo-Check Analyzer I (Diatech). Endotoxin levels were calculated from kinetic graphs, and the cutoff value was set to 0.005 EU/mL.
The QoL change in the course of treatment was determined at the baseline and the 5th week of radiotherapy using the Korean version of the cancer-specific module of the European Organization for Research and Treatment of Cancer (EORTC) QLQ C-30 questionnaire [13]. The Korean EORTC QLQ C-30 was developed by EORTC using a rigorous translation and back translation process, and demonstrated as a valid instrument for evaluating Korean-speaking patients with cancer. Korean version of EORTC QLQ C-30 have the questionnaire incorporating five functional scales (physical, role, cognitive, emotional, and social), three symptom scales (fatigue, pain, and nausea and vomiting), a global health and QoL scale, and single items for the assessment of additional symptoms commonly reported by cancer patients (e.g., dyspnea, appetite loss, sleep disturbance, constipation, and diarrhea), as well as the perceived financial impact of the disease and treatment. QLQ-C30 adopted a self-rating scale for patient completion at baseline and the 5th week of treatment. All scale and item scores were transformed into a scale of 0-100. High scores for a functional scale and global health status reflect a high and healthy level of functioning and a high QoL, respectively. However, a high score for a symptom scale/item reflects a high level of symptoms and problems.
All data are expressed as means±standard error, and we used Sigma Stat software (Systat Software Inc., San Jose, CA, USA). Weekly measured data were compared to baseline values by using the paired t-test. We used the paired t-test just to ascertain which session shows the significant change compared to the baseline. Paired t-test was also used in comparing the changes in symptoms between at baseline and at final treatment. The relationship between biologic markers and patient's symptoms was analyzed via Kendall tau. The p-values less than 0.05 were considered statistically significantly different.
The mean basal blood GSH for 12 patients was 894.6±90.5 M (range, 549.0 to 1,408.2 M) and no significant changes were noted in the level of GSH during radiotherapy. The mean value of GSSG, which is the oxidized form of GSH and reflects the extent of oxidative stress, was 27.2±10.8 M at baseline. During the course of radiotherapy, GSSG was significantly high at the 2nd (85.7±17.2, p=0.01), 3rd (72.6±13.7, p=0.018) and 4th (76.6±16.3, p=0.01) weeks of radiotherapy, and returned to basal levels at the 5th week of treatment (27.4±34.4). The GSH/GSSG ratio after treatment was reduced dramatically at the 2nd (12.9±5.6, p=0.026), 3rd (11.5±3.6, p=0.045), and 4th (14.6±5.7, p=0.045) weeks of radiotherapy (Fig. 1). Although it was not statistically significantly different, the GSH/GSSG ratio after the 5th week of treatment (27.6±3.6) never fully recovered to baseline (46.1±12.3).
The cutoff value of plasma endotoxin was set at 0.005 EU/mL. The mean value of plasma endotoxin for 12 patients was 0.0045±0.0012 EU/mL and 2 out of 12 patients (16.7%) evidenced positive values prior to treatment, which is defined as values in excess of the cutoff value. The mean endotoxin level for all patients evidenced an increasing tendency and persisted during radiotherapy (Fig. 2A). Additionally, the number of patients with greater than the cutoff value of endotoxin was increased at the end of radiotherapy (8/12) (Fig. 2B).
The mean patient scores are provided in Table 2. In functioning scales of the Korean version of EORTC QLQ-C30, the global health status item (54.17±4.81) was the lowest score in patient subjects prior to radiotherapy. Among symptom scales and single-item scales, the fatigue score showed the highest value (40.74±4.06) at pre-radiotherapy. In paired t-test analysis between different items at baseline, radiotherapy induced a significant change in the score of global health status (p=0.049) in functioning scales, and nausea/vomiting apparently worsened (p=0.019) in the symptom scales. When compared to the baseline in single-item scales, scores for appetite loss and diarrhea were increased significantly, by 183.3% (p=0.045) and 850% (p<0.001), respectively.
Significant symptom changes in nausea/vomiting and diarrhea were observed pre- and post-radiotherapy in this study. Such symptoms evidenced no correlation with biologic markers of oxidative stress, including GSH, GSSG, and GSH/GSSG. However, the change in the level of endotoxin was associated significantly with nausea/vomiting (p=0.001) (Fig. 3A), but not with diarrhea (p=0.196) (Fig. 3B).
This study aimed to assess the influence of pelvic radiotherapy on biologic markers of oxidative stress and plasma endotoxin levels, and to assess the relationship between the changes of such factors and radiotherapy-related adverse events. A few previous studies have implied that systemic oxidative burden may increase as the result of radiotherapy with or without concomitant chemotherapy [14-19]. We measured blood GSH and GSSH, which may serve as a good indicator of the oxidative stress of both the body and the tumor [20]. This study was unable to detect any significant changes in the levels of GSH during radiotherapy. However, unlike what was found with GSH, GSSG accumulated and GSH/GSSG was reduced under increased oxidative stress conditions. Although GSH is a ubiquitous cellular antioxidant and a highly sensitive indicator of cell functionality and viability under physiological and pathological conditions, GSH is known to be recycled back by GSH reductase [21]. Transient reduction and immediate recovery of GSH levels may not be accurate indicators of oxidative stress status. Therefore, according to this study, the value of GSSG and the ratio of GSH/GSSG may prove to be more useful indicators of oxidative stress status than that of GSH in patients treated with radiotherapy. Nevertheless, it is not clear why GSSG level returned to basal levels at the 5th week of treatment. It might be partly explained by the hypothesis that GSSG is known to be reduced back to GSH by GSH reductase using NADPH. If reactive oxygen species increases, it oxidizes our body and simultaneously induces multiple antioxidative systems, which might compensate the oxidative burden and recycle the GSH system.
We were unable to come to any clear conclusions regarding the correlation between changes in GSH/GSSG ratio and subjective symptoms. The tendency to return to basal levels at the 5th week made it quite difficult to analyze the relevant relationships, considering that the severity of radiotherapy-related adverse effects generally increases from the beginning to the end of radiotherapy. Additionally, the degree and pattern of changes in GSH/GSSG ratio differed among individual cases. The GSH/GSSG ratio was observed to recover at the middle or final stage in some patients, while others did not recover, evidencing sustained oxidations of the GSH-GSSG system. Therefore, it remains to be clearly determined whether oxidative stress, which was determined as a GSH/GSSG ratio in our study, is a simply transient therapeutic toxicity associated with radiotherapy, or if this impairment of redox balance serves as an early indicator in the development of treatment-related adverse events [22]. Diminishing oxidative stress has been recognized as an important therapeutic approach, and thus antioxidants are frequently taken in conjunction with conventional anticancer treatment to reduce a variety of complications. Some investigators who are concerned about the role of oxidative stress in the development of various complications have attempted to manage this oxidative stress with a variety of antioxidant interventions during the administration of cancer therapies [21,23]. However, opponents insist that antioxidant intervention may reduce the therapeutic effects of anticancer therapies, because the anti-cancer effects of chemotherapy or radiotherapy is substantially mediated by oxidative stress via the generation of free radicals, and thus the use of antioxidant may occasionally interfere with the effects of anticancer treatment. Until now, there has been a decided lack of evidence not only with regard to the clinical efficacy of antioxidants, but also the diminishing effect against anticancer therapy. In particular, very little information is currently available regarding oxidative stress and the efficacy of antioxidant intervention from a radiotherapy perspective. Therefore, further investigations into the clinical significance of oxidative stress will be necessary to clearly assess the feasibility of the clinical use of antioxidative intervention.
Endotoxins, another biologic marker, was measured during pelvic radiotherapy. Pelvic radiotherapy can damage intestinal mucosal cells, by which endotoxin can enter into the blood via the degradation of the gut permeability barrier. Endotoxins are a component of the outer membrane of gram-negative bacteria and is known to lead to innate immune system when administered to rodents or humans [24]. In this study, endotoxins evidenced a tendency to increase, and the number of patients with a positive endotoxin value during radiotherapy tended to increase during radiotherapy. Among subjective symptoms determined over the course of treatment, nausea/vomiting and diarrhea were observed at dramatic levels at the end of radiotherapy. A close correlation between nausea/vomiting and the change in the plasma endotoxin level was observed. Although this result does not necessarily mean that the endotoxin directly induces nausea/vomiting or diarrhea, endotoxin might be one of the critical factors that induce radiotherapy-related adverse events. In the rat model, the levels of endotoxin increased significantly in irradiated rats when compared to healthy controls, and radiation enteritis was related closely to bacteremia and endotoxinemia. High doses of endotoxin (>1 ng/kg) administration can induce flu-like symptoms, and low doses of endotoxin administration (<1 ng/kg) in human subjects may induce inflammatory processes, which result in fatigue, reduced appetite, and cognitive impairments [25]. Also, a probiotic interventional study by Seal et al. [25] reported that the endotoxin levels increased significantly in irradiated rats and probiotics reduce radiotherapy-induced enteritis along with a reduction in endotoxin levels. Therefore, further investigations to enroll more patients and interventional studies with probiotics, which are known to reduce endotoxins, will be required to elucidate clearly the relationship between endotoxins and radiotherapy-related enteritis.
This study has a major limitation, which must be considered when interpreting the results. Although all 12 patients received same radiation portal, i.e. whole pelvic radiotherapy, with median total dose of 50.4 Gy, the small number of patients made it difficult to analyze sufficiently the effects of changes in biological markers on radiotherapy-related symptoms.
Additionally, we were unable to effectively assess the effects of the addition of chemotherapy to pelvic radiotherapy on the outcomes of this study, although it is known that the addition of chemotherapy is one of the most important factors that might influence treatment-related complications. Although 2 of 3 patients treated with adjuvant radiotherapy alone without chemotherapy showed significant changes in the level of endotoxins during radiotherapy, and these 2 patients experienced nausea and vomiting, it may not be possible to draw confirmatory conclusions for the relationship between the change in the level of endotoxin and radiotherapy-related adverse effects. However, this was a pilot study to test the hypothesis whether pelvic radiotherapy with or without chemotherapy could change some biologic markers that might be related with radiotherapy and undoubtedly showed that pelvic radiotherapy oxidized the GSH redox system and increased plasma endotoxin.
Based on these results, further investigations containing interventional and longitudinal studies will be required to assess the effects of the changes in oxidative stress markers and endotoxin on radiotherapy-related adverse events. Also, the clinical application of these findings has to be considered to minimize radiotherapy-related adverse events and to improve QoL in cancer patients.
Figures and Tables
 | Fig. 1Glutathione (GSH)/oxidized glutathione (GSSG) ratio during radiotherapy. GSH/GSSG ratio=GSH/GSSG. |
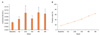 | Fig. 2Blood endotoxin level (A) and positive rate (B) in patient subjects during radiotherapy. Endotoxin was expressed as mean±standard error. Endotoxin level between baseline and at 5th week of treatment was statistically significant (p<0.05) according to the results of the t-test. |
 | Fig. 3The change in the level of endotoxin was associated significantly with nausea/vomiting (p=0.001; (A) arrow head: 3 patients overlapped), but not with diarrhea (p=0.196; (B) arrow: 2 patients overlapped). Endotoxin final-baseline, difference in the endotoxin values between final and baseline week; Nausea and vomiting severity, difference in the severity of nausea and vomiting between final and baseline week; Diarrhea severity, difference in the severity of diarrhea between final and baseline week. |
Table 2
Result of Korean version of EORTC QLQ-C30 questionnaire at baseline and 5th week of radiotherapy

Values are presented as mean±standard error.
EORTC, European Organization for Research and Treatment of Cancer; QLQ, quality of life questionnaire.
*p-value was determined by paired t-test between baseline and final treatment. †Scores range from 0 to 100, with a higher score representing a greater degree of symptoms.
ACKNOWLEDGMENTS
This work was supported by SNUH CRI grant (0420100900) and also supported by nonprofit research grants for integrative medicine from SK Holdings and late Chairman Jong-Hyun Choi.
References
1. Kim HJ, Ha SW, Wu HG. Treatment outcomes and prognostic factors in uterine cervical cancer patients treated with postoperative extended field radiation therapy. J Gynecol Oncol. 2009. 20:227–231.
2. Kim YH, Chung HH, Kim JW, Park NH, Song YS, Kang SB. Prognostic significance of neutropenia during adjuvant concurrent chemoradiotherapy in early cervical cancer. J Gynecol Oncol. 2009. 20:146–150.
3. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993. 85:365–376.
4. Trask PC, Hsu MA, McQuellon R. Other paradigms: health-related quality of life as a measure in cancer treatment: its importance and relevance. Cancer J. 2009. 15:435–440.
5. Andreyev J. Gastrointestinal complications of pelvic radiotherapy: are they of any importance? Gut. 2005. 54:1051–1054.
6. Hurley JC. Endotoxemia: methods of detection and clinical correlates. Clin Microbiol Rev. 1995. 8:268–292.
7. Maxwell A, Gaffin SL, Wells MT. Radiotherapy, endotoxaemia, and nausea. Lancet. 1986. 1:1148–1149.
8. Richie JP Jr, Skowronski L, Abraham P, Leutzinger Y. Blood glutathione concentrations in a large-scale human study. Clin Chem. 1996. 42:64–70.
9. Pastore A, Federici G, Bertini E, Piemonte F. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta. 2003. 333:19–39.
10. Araujo AR, Saraiva ML, Lima JL. Determination of total and oxidized glutathione in human whole blood with a sequential injection analysis system. Talanta. 2008. 74:1511–1519.
11. Kim JH, Kim HJ, Hong S, Wu HG, Ha SW. Post-hysterectomy radiotherapy in FIGO stage IB-IIB uterine cervical carcinoma. Gynecol Oncol. 2005. 96:407–414.
12. Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969. 27:502–522.
13. Yun YH, Park YS, Lee ES, Bang SM, Heo DS, Park SY, et al. Validation of the Korean version of the EORTC QLQ-C30. Qual Life Res. 2004. 13:863–868.
14. Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006. 52:601–623.
15. Benzie IF, Wachtel-Galor S. Vegetarian diets and public health: biomarker and redox connections. Antioxid Redox Signal. 2010. 13:1575–1591.
16. Rossi R, Dalle-Donne I, Milzani A, Giustarini D. Oxidized forms of glutathione in peripheral blood as biomarkers of oxidative stress. Clin Chem. 2006. 52:1406–1414.
17. Chen Y, Jungsuwadee P, Vore M, Butterfield DA, St Clair DK. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol Interv. 2007. 7:147–156.
18. McGough C, Baldwin C, Frost G, Andreyev HJ. Role of nutritional intervention in patients treated with radiotherapy for pelvic malignancy. Br J Cancer. 2004. 90:2278–2287.
19. Mossman KL, Henkin RI. Radiation-induced changes in taste acuity in cancer patients. Int J Radiat Oncol Biol Phys. 1978. 4:663–670.
20. Afzal M, Afzal A, Jones A, Armstrong D. A rapid method for the quantification of GSH and GSSG in biological samples. Methods Mol Biol. 2002. 186:117–122.
21. Ladas E, Kelly KM. The antioxidant debate. Explore (NY). 2010. 6:75–85.
22. Pan H, Mukhopadhyay P, Rajesh M, Patel V, Mukhopadhyay B, Gao B, et al. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J Pharmacol Exp Ther. 2009. 328:708–714.
23. Ladas EJ, Jacobson JS, Kennedy DD, Teel K, Fleischauer A, Kelly KM. Antioxidants and cancer therapy: a systematic review. J Clin Oncol. 2004. 22:517–528.
24. DellaGioia N, Hannestad J. A critical review of human endotoxin administration as an experimental paradigm of depression. Neurosci Biobehav Rev. 2010. 34:130–143.
25. Seal M, Naito Y, Barreto R, Lorenzetti A, Safran P, Marotta F. Experimental radiotherapy-induced enteritis: a probiotic interventional study. J Dig Dis. 2007. 8:143–147.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download