Abstract
Objective
It is clear that uterine carcinosarcomas and uterine papillary serous carcinomas (UPSC) have an adverse impact on outcome, but whether carcinosarcomas are worse than UPSC is unclear. The purpose of this study is to compare the pathology, survival, and disease recurrence of patients with carcinosarcomas to patients with UPSC.
Methods
The medical records of patients diagnosed with carcinosarcomas and UPSC between 1996 and 2009 at Samsung Medical Center were retrospectively analyzed. Information from pathology reports, site of relapse, time to recurrence, and death was obtained. The survival analysis was performed using the Kaplan-Meier method.
Results
Thirty seven patients with carcinosarcomas and 38 patients with UPSC were identified during the study period. There was no significant difference in clinical characteristics including age, body mass index, proportion with advanced stage disease, rate of optimal debulking, and adjuvant treatment used. In addition, the pathology showed no significant difference in tumor size, myometrial involvement, lymphovascular invasion, peritoneal cytology, cervical invasion, and lymph node involvement. Patients with carcinosarcomas had similar patterns of relapse as the patients with UPSC. There was no difference in the progression-free and overall survival between the carcinosarcomas and UPSC patients (p=0.804 and p=0.651, respectively).
Endometrial cancer is the most common gynecologic malignancy in women in the United States [1]. In 2009, an estimated 42,160 new cases and 7,780 cancer-related deaths were anticipated [1]. Certain clinical/pathologic characteristics have been shown to be significant predictors of outcome such as tumor grade. The 5-year survival rate for women with stage IA grade 1 cancer exceeds 90%, whereas it is only 69% for women with stage IA grade 3 cancer [2]. In addition to grade, the histopathological types of the endometrial cancer influence the outcome. For example, patients with uterine papillary serous carcinomas (UPSC) or carcinosarcomas that was termed malignant mixed mullerian tumor are thought to have a worse outcome than those with endometrioid adenocarcinomas of the uterus. The poor outcome in these patients is often attributed to the more advanced stage at the time of diagnosis and disease relapse in the upper abdomen [3,4].
What is unclear from the literature is which one is worse between carcinosarcomas and UPSC. This is partly because of the low incidence of these tumors. To the best of our knowledge, there is no report which has compared the two tumors directly. This study was undertaken to compare the pathology, survival, and disease recurrence of patients with carcinosarcomas to patients with UPSC.
The study population included patients with primary uterine carcinosarcomas and UPSC treated at the Samsung Medical Center (SMC, Korea) between April 1996 and March 2009. Patients that were referred to our center for recurrent disease that developed following primary treatment at another hospital, or those that had incomplete medical records were excluded from the analysis. In addition, mixed form carcinomas, regardless of the percent of carcinosarcomas or papillary serous component, were excluded from this study. Data were retrieved from the patient's records and death certificates. All pathology specimens were reassessed independently for their histopathological type, composition, and tumor characteristics, by two pathologists specializing in gynecology. Cases in which the diagnosis was not of the same opinion were also excluded from this series. All surgical and pathologic data, including histology, size, location, uterine variables, lymph node involvement (by areas: pelvic, para-aortic), and other organ involvement (peritoneum, omentum, bowel, diaphragm, spleen, liver), as well as cytology, were reviewed. Stage assignment was made according to the International Federation of Gynecology and Obstetrics (FIGO) surgical staging criteria reported in 1988 [5]. Information regarding treatment, including surgery, chemotherapy and/or radiation therapy and follow-up was collected.
The patients were considered to be appropriately surgically staged if a lymphadenectomy was performed in patients with disease stages I through III. In patients with demonstrable intra-abdominal or distant metastasis (stage IV disease), a biopsy of the extra-pelvic disease site was considered sufficient for surgical staging. The amount of residual disease after surgery, as abstracted from the surgical report, was recorded as optimal or suboptimal debulking. Optimal debulking was considered if all of the cancer had been removed with the exception of residual nodules that measured no more than 1 cm in the maximum diameter.
Determination of patient outcomes, including the sites and number of treatment failures as well as the causes of death, were the primary study objectives. The specific sites of failure were categorized as local or distant. The dominant histological feature of disease recurrence was identified when available. A vaginal recurrence (out of local) was defined when the disease was diagnosed during follow-up at the proximal vaginal site. Treatment failures detected in the central pelvis, pelvic side wall, pelvic and/or para-aortic lymph nodes were considered local recurrences. Distant recurrences were defined as disease recurring in the upper abdomen or in extra-abdominal sites after initial treatment. Recurrences lacking pathological documentation were determined based on patient symptoms, imaging studies, elevated CA 125 levels and/or autopsy information.
The progression-free survival (PFS) was defined as the time (months) from surgery to the date of the last follow-up or recurrence, and was based on imaging and tumor marker elevation. The overall survival (OS) was calculated from the day of surgery to the date of death or last contact. The PFS and OS were estimated on the basis of Kaplan-Meier curves. The statistical analysis was performed with SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). A p-value less than 0.05 was considered statistically significant.
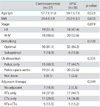
During the study period, 37 patients with carcinosarcomas and 38 patients with UPSC met the inclusion criteria. The clinical characteristics of the study population are listed in Table 1. The mean age of carcinosarcomas group was 57.7 (±11.6 years) and that of UPSC was 59.1 (±7.6 years). There was no difference in age and body mass index (BMI) (p=0.523 and p=0.633, respectively). The distribution of advanced stage disease (III and IV) was 48.6% and 52.6% for carcinosarcomas and UPSC, respectively. The rate of optimal debulking was 81.1% and 84.2% for carcinosarcomas and UPSC, respectively. Most patients in both groups underwent comprehensive surgical staging, defined as peritoneal cytology and pelvic and/or para-aortic lymph node dissection; 91.9% of the carcinosarcomas, and 97.4% of the UPSC. There was no statistical difference in the proportion of advanced stage disease, cases with optimal debulking and lymph node dissection between the two groups (p=0.819, p=0.720, and p=0.335, respectively). Furthermore, adjuvant therapy after primary surgery did not significantly differ between the two groups (p=0.349).
Table 2 shows the pathological characteristics. There was no difference in the tumor size in the uterus, myometrial involvement, lymphovascular invasion, peritoneal cytology, cervical invasion, and lymph node involvement between the two groups (p=0.103, p=0.734, p=0.416, p=0.358, p=0.170, and p=0.449, respectively).
Among the 75 patients, 28 (37.3%) had a recurrence; 15 in the carcinosarcomas and 13 in the UPSC group (Table 3). The sites of recurrence included the vagina in 3 (4.0%), pelvis in 13 (17.3%), and more distant sites in 12 patients (16.0%). Eight of the 28 patients with a recurrence had more than one site of relapse. Sixteen patients (21.3%) had an isolated local recurrence. The most common site for distant recurrence was the lungs (5 of 12 patients) followed by intra-abdominal dissemination (4 of 12 patients), the liver, and the bones. In the four patients with intra-abdominal dissemination, two had carcinosarcomas and two had UPSC. There was no significant difference between the two groups with regard to local relapse; 60.0% (9 of 15) in the carcinosarcomas group; 54 % (7 of 13) in the UPSC group (p=0.807).
Fig. 1 shows the PFS and OS in patients for all disease stages. In patients with carcinosarcomas and UPSC, the five-year PFS was 42.5% and 50.7%, respectively; there was no significant difference between carcinosarcomas and UPSC (p=0.804). The results were similar for both early stage (stage I and II; p=0.774) and advanced stage tumors (stage III and IV; p=0.918). In patients with carcinosarcomas and UPSC, the five-year OS was 61.0% and 66.8%, respectively; there was no significant difference between the two groups (p=0.651).
Given that carcinosarcomas and UPSC are rare uterine neoplasms, comparative studies of their clinical behavior and outcomes are limited. Generally, patients with carcinosarcomas are thought to have a poorer outcome than patients with high-risk endometrial carcinomas such as UPSC [6-8]; this is because of the traditional classification that carcinosarcomas had been regarded as a subtype of uterine sarcoma. However, in this study comparing UPSC with carcinosarcomas, the findings showed that patients with UPSC had a similar outcome compared to patients with carcinosarcomas. Considering the rarity of these tumors, our study is meaningful and it may contribute to better understanding of the behavior of these tumors.
However, there are some differences between the results of this study and prior studies. The major reason is that the current study is a direct comparison of carcinosarcomas versus UPSC alone for clinicopathologic outcomes. Amant et al. compared carcinosarcomas with non-endometrioid carcinomas in their study [6]. UPSC and clear cell carcinomas were included in the non-endometrioid carcinomas. The results showed that patients with carcinosarcomas had a poorer outcome when compared to patients with non-endometrioid carcinomas. According to the FIGO sixth annual report on the results of treatment for gynecological cancer [9], however, patients with UPSC had a less favorable survival outcome than patients with clear cell carcinomas. The 5-year survival rate was 52.6% for UPSC, compared to 62.5% for clear cell carcinomas. When only patients with stage I disease were analyzed, the 5-year survival rate was 79.9% for UPSC, compared to 85.1% for clear cell carcinomas. Such a comparison including the clear cell carcinomas in the UPSC group may magnify the differences between carcinosarcomas and UPSC. Vaidya et al. classified carcinosarcomas as cases and patients with high-risk endometrial carcinomas as controls [8]. Grade 3 endometrioid adenocarcinomas, clear cell carcinomas, and UPSC were included in the high-risk endometrial carcinomas. As a result, patients with carcinosarcomas had a poorer prognosis than patients with high-risk endometrial carcinomas. The difference between the groups was magnified due to the grade 3 endometrioid adenocarcinomas that were included in the cases; these tumors have been reported to have an adverse impact on outcomes with UPSC or carcinosarcomas in the literature [6,7,10].
Of interest were the similarities in the pattern of recurrence between the common epithelial endometrial carcinomas and the carcinosarcomas/UPSC. In a 1984 report on 379 patients with recurrent endometrial cancer at the Norwegian Radium Hospital, from 1960 to 1976 [11], the sites of recurrence were local in 190 patients (50.1%) and distant in 189 patients (49.9%). Although the site of recurrence has not been reported for carcinosarcomas, for UPSC it was local in six patients (46%) and distant in seven patients (54%) according to a study reported by Alektiar et al. [12]. The data from this study showed that the carcinosarcomas was local in nine patients (60%) and distant in six patients (40%), and that the UPSC was local in seven patients (54%) and distant in six patients (46%). The sites of distant recurrence were similar for both study groups. The common sites of distant recurrence are the lungs and abdomen in the common epithelial endometrial carcinomas [13]. According to Amant et al. [6] and Alektiar et al. [12], the most common site in both groups is the lungs; this is consistent with the results of this study.
The findings of this study raise the question of why the patients with carcinosarcomas, traditionally regarded as a subtype of uterine sarcoma, had similar outcomes to the patients with UPSC. Recent research suggests that carcinosarcomas are monoclonal in origin, with the sarcomatous component representing dedifferentiation of the carcinomatous portion [14]. However, the carcinomatous origins are thought to be the primary characteristic. Histologically, tumor emboli within lymphovascular channels and metastases primarily consist of carcinomatous components [15]. Further evidence that carcinosarcomas develops from a single stem cell comes from studies examining the immunohistochemical expression of the p53 protein. The p53 protein expression was found in both the carcinomatous and sarcomatous portions of the tumor [16, 17]. Carcinosarcomas has similar risk factors as endometrial carcinomas. Both neoplasms are associated with obesity, nulliparity, and exogenous estrogen use [18]. Therefore, carcinosarcomas are now classified as carcinomas rather than a mixture of carcinoma and sarcoma [19]. In addition, the epidemiology, risk factors, and clinical behavior associated with carcinosarcomas suggest a closer relationship to endometrial carcinomas than to sarcoma.
The limitations of this study include the following. The study was retrospective with inherent associated biases. Over the 14 years of the study, the treatment protocols have changed. Furthermore, most prior studies on endometrial cancer have been performed on the Caucasian population [20,21]. The results of this study may be specific only to Asians; the survival differences associated with histopathological types vary considerably with racial background according to the study reported by Sherman and Devesa [22]. In addition, the number of patients was relatively small, which may have affected the results. Not all patients in this series underwent pelvic and para-aortic lymph node dissection, although most patients underwent pelvic dissection (91.9% in carcinosarcomas and 97.3% in UPSC); therefore, the data from this study may not be applicable to patients that undergo routine dissection of pelvic and para-aortic lymph nodes.
In conclusion, the pathological features, strategies for adjuvant management, disease recurrence and death, as well as sites of relapse were similar in patients with carcinosarcomas and UPSC. Additional follow-up on a larger number of patients is needed to confirm the data from this study and expand our knowledge on these two malignancies.
Figures and Tables
Fig. 1
Progression-free survival for all stages (A) and overall survival for all stages (B). UPSC, uterine papillary serous carcinomas.

References
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009. 59:225–249.
2. Creasman WT, Odicino F, Maisonneuve P, Beller U, Benedet JL, Heintz AP, et al. Carcinoma of the corpus uteri. J Epidemiol Biostat. 2001. 6:47–86.
3. Goff BA, Kato D, Schmidt RA, Ek M, Ferry JA, Muntz HG, et al. Uterine papillary serous carcinoma: patterns of metastatic spread. Gynecol Oncol. 1994. 54:264–268.
4. Nielsen SN, Podratz KC, Scheithauer BW, O'Brien PC. Clinicopathologic analysis of uterine malignant mixed mullerian tumors. Gynecol Oncol. 1989. 34:372–378.
5. Gal D, Recio FO, Zamurovic D. The new International Federation of Gynecology and Obstetrics surgical staging and survival rates in early endometrial carcinoma. Cancer. 1992. 69:200–202.
6. Amant F, Cadron I, Fuso L, Berteloot P, de Jonge E, Jacomen G, et al. Endometrial carcinosarcomas have a different prognosis and pattern of spread compared to high-risk epithelial endometrial cancer. Gynecol Oncol. 2005. 98:274–280.
7. Bland AE, Stone R, Heuser C, Shu J, Jazaeri A, Shutter J, et al. A clinical and biological comparison between malignant mixed mullerian tumors and grade 3 endometrioid endometrial carcinomas. Int J Gynecol Cancer. 2009. 19:261–265.
8. Vaidya AP, Horowitz NS, Oliva E, Halpern EF, Duska LR. Uterine malignant mixed mullerian tumors should not be included in studies of endometrial carcinoma. Gynecol Oncol. 2006. 103:684–687.
9. Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri: FIGO 6th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006. 95:Suppl 1. S105–S143.
10. Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006. 94:642–646.
11. Aalders JG, Abeler V, Kolstad P. Recurrent adenocarcinoma of the endometrium: a clinical and histopathological study of 379 patients. Gynecol Oncol. 1984. 17:85–103.
12. Alektiar KM, McKee A, Lin O, Venkatraman E, Zelefsky MJ, McKee B, et al. Is there a difference in outcome between stage I-II endometrial cancer of papillary serous/clear cell and endometrioid FIGO Grade 3 cancer? Int J Radiat Oncol Biol Phys. 2002. 54:79–85.
13. Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991. 40:55–65.
14. McCluggage WG. Uterine carcinosarcomas (malignant mixed Mullerian tumors) are metaplastic carcinomas. Int J Gynecol Cancer. 2002. 12:687–690.
15. Silverberg SG, Major FJ, Blessing JA, Fetter B, Askin FB, Liao SY, et al. Carcinosarcoma (malignant mixed mesodermal tumor) of the uterus: a Gynecologic Oncology Group pathologic study of 203 cases. Int J Gynecol Pathol. 1990. 9:1–19.
16. Mayall F, Rutty K, Campbell F, Goddard H. p53 immunostaining suggests that uterine carcinosarcomas are monoclonal. Histopathology. 1994. 24:211–214.
17. Szukala SA, Marks JR, Burchette JL, Elbendary AA, Krigman HR. Co-expression of p53 by epithelial and stromal elements in carcinosarcoma of the female genital tract: an immunohistochemical study of 19 cases. Int J Gynecol Cancer. 1999. 9:131–136.
18. Zelmanowicz A, Hildesheim A, Sherman ME, Sturgeon SR, Kurman RJ, Barrett RJ, et al. Evidence for a common etiology for endometrial carcinomas and malignant mixed mullerian tumors. Gynecol Oncol. 1998. 69:253–257.
19. Ronnett B, Zaino R, Ellenson L. Kurman R, editor. Endometrial carcinoma. Blaustein's pathology of the female genital tract. 2002. 5th ed. New York: Springer-Verlag;460–501.
20. Madison T, Schottenfeld D, Baker V. Cancer of the corpus uteri in white and black women in Michigan, 1985-1994: an analysis of trends in incidence and mortality and their relation to histologic subtype and stage. Cancer. 1998. 83:1546–1554.
21. Matthews RP, Hutchinson-Colas J, Maiman M, Fruchter RG, Gates EJ, Gibbon D, et al. Papillary serous and clear cell type lead to poor prognosis of endometrial carcinoma in black women. Gynecol Oncol. 1997. 65:206–212.
22. Sherman ME, Devesa SS. Analysis of racial differences in incidence, survival, and mortality for malignant tumors of the uterine corpus. Cancer. 2003. 98:176–186.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download