Abstract
Chylous ascites is an uncommon postoperative complication of gynecological surgery. We report a case of chylous ascites following a robotic lymph node dissection for a cervical carcinoma. A 38-year-old woman with IB2 cervical adenocarcinoma with a palpable 3 cm left external iliac lymph node was taken to the operating room for robotic-assisted laparoscopic pelvic and para-aortic lymph node dissection. Patient was discharged on postoperative day 2 after an apparent uncomplicated procedure. The patient was readmitted the hospital on postoperative day 9 with abdominal distention and a CT-scan revealed free fluid in the abdomen and pelvis. A paracentesis demonstrated milky-fluid with an elevated concentration of triglycerides, confirming the diagnosis of chylous ascites. She recovered well with conservative measures. The risk of postoperative chylous ascites following lymph node dissection is still present despite the utilization of new technologies such as the da Vinci robot.
Chylous ascites is defined as a milky-appearing peritoneal fluid that is rich in triglycerides, due to the presence of thoracic or intestinal lymph in the abdominal cavity [1]. The clinical presentation and physical exam findings associated with this postoperative complication are often non-specific and typically include abdominal distention, indigestion, nausea, and vomiting. Acute abdomen findings, including a positive rebound sign, can also occur in the setting of chylous peritonitis [2]. Most symptomatic patients with postoperative chylous ascites present a few days to one month after surgery, most commonly after approximately 1 week [3].
We managed a case of postoperative chylous ascites associated with a robotic-assisted laparoscopic pelvic and para-aortic lymph node dissection for a cervical carcinoma that was readmitted to the hospital on postoperative day number 9 with abdominal ascites. To our knowledge, this represents the first case of its kind in the literature.
A 38-year-old woman, G2, P2, presented with a 6 month history of metrorrhagia and dyspareunia. Prior to referral, she had undergone a hysteroscopic resection of an 8 cm endocervical mass. Histopathology revealed a poorly-differentiated endocervical adenocarcinoma which was confirmed by immunohistochemical stains. The physical examination was otherwise within normal limits, and the body mass index was 16.4. The patient was staged clinically as IB2 cervical adenocarcinoma. Subsequent positron emission tomographic scan showed a hypermetabolic focus in the left external iliac region.
The patient returned to the operating room for planned robotic assisted lymph node dissection. Examination under anesthesia revealed a grossly normal cervix, and a palpable 3 cm left pelvic sidewall mass. A robotic-assisted laparoscopic pelvic and para-aortic lymph node dissection was performed. The palpable 3 cm left external iliac lymph node was identified just distal to the bifurcation of the common iliac artery. The adjacent lymphatics of this enlarged node were first secured with hemoclips and the node was resected using sharp dissection and monopolar electrosurgery. Pathology revealed poorly-differentiated metastatic adenocarcinoma with focal mucinous features. Complete bilateral pelvic and paraaortic lymph node dissection was completed and no additional metastatic sites were identified (1/12). She was discharged home on the same day of surgery.
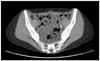
On postoperative day 9, the patient was readmitted to the hospital due to abdominal distention and early satiety. Vital signs were within normal limits. On physical exam, the abdomen was soft and distended with a positive fluid-wave sign. There was mild tenderness on deep palpation but no rebound tenderness. Computed tomography (CT) scan revealed free fluid with several hemoclips in the left pelvic area corresponding to the site of resection of the enlarged lymph node. There was no hydronephrosis and an intact urinary bladder was noticed (Fig. 1). Diagnostic paracentesis was performed and 400 mL of milky-fluid was obtained. Ascitic fluid analysis was significant for lymphocytes of 98%, and triglycerides of 236 mg/dL, confirming the diagnosis of chylous ascites. The patient was initially placed on IV hydration, total parental nutritional (TPN) support and restricted from oral intake (NPO). She was later managed with low fat diet, medium chain free fatty acids, and octreotide treatment. The patient was discharged home on the same treatment and followed with serial imaging studies until the ascites resolved six weeks after the initial diagnosis of chylous ascites.
After resolution of the chylous ascites, the patient completed chemoradiation therapy, during which she developed diarrhea but no grade 3-4 hematologic toxicity. The patient has remained well with no evidence of disease 40 months after the diagnosis of her metastatic IB2 cervical adenocarcinoma (AJCC, IIIB; TNM, T1BN1M0). Recent CT scan of the abdomen and pelvis showed no evidence of disease with only trace amount of pelvic fluid.
Chylous ascites is defined as a milky-appearing peritoneal fluid that is rich in triglycerides, due to the presence of thoracic or intestinal lymph in the abdominal cavity [1]. It is an uncommon complication of gynecologic surgery and to our knowledge this is the first report in the literature of chylous ascites following a robotic lymph node dissection.
The clinical presentation and physical exam findings of chylous ascites are frequently non-specific and typically include abdominal distention, indigestion, nausea, and vomiting. Acute abdomen findings, including a positive rebound sign, can also occur in the setting of chylous peritonitis [2]. Most symptomatic patients with postoperative chylous ascites present a few days to one month after surgery, most commonly after approximately 1 week [3].
The patient in the current case was readmitted to the hospital with clinical signs and symptoms that are typically seen in chylous ascites, including abdominal distention with a positive fluid-wave sign on physical exam. Of note, atypical presentations have also been reported in the literature including a case of vulvar edema. The mechanism of chyle accumulation in this specific case was believed to be that the unhealed puncture tract allowed ascites to travel through the subcutaneous tissue and accumulate in the labia majora [4].
When the clinical presentation suggests chylous ascites, a paracentesis with fluid analysis is typically required to establish the diagnosis [5]. The ascitic fluid in chylous ascites, has a milky appearance and an elevated triglyceride level (>200 mg/dL), which are required to establish the diagnosis [6].
In their review of the literature, Aalami et al. [3], reported a successful conservative management of 67% of the cases, with 33% of patients requiring a surgical intervention. These procedures included a laparotomy with ligation of the leak or placement of a peritoneal-venous shunt.
Conservative management typically includes palliative paracentesis, initiation of a low-fat diet with medium-chain triglycerides with or without somatostatin or octreotide (somatostatin analog) treatment. Successful treatment with octreotide (100 micrograms subcutaneously every 8 hours) or somatostatin (continuous intravenous administration at 6 mg/day) has been reported in the literature [6,7]. Somatostatin decreases the intestinal fat absorption and reduces both the triglyceride level and lymphatic flow in the major lymphatic vessels [8]. The medium-chain triglycerides, in contrast with long-chain triglycerides, are used for supplementation since they are absorbed directly into the intestinal cells and transported directly to the liver, which reduces the net production and flow of chyle [9].
For intractable cases after a trial of conservative management of chylous ascites, a number of different surgical approaches with various grades of complexity have been successfully described in the non-gynecologic surgical literature. The initial surgical approach often recommended is an exploratory laparoscopy or laparotomy, in which the site of the leak may be identified and then clipped or closed with fibrin glue [10-12]. Other treatment options that have been described include placement of a peritoneal-venous shunt [13,14] or a peritoneal-atrial shunt, for the cases in which the former might be contraindicated [15].
The risk of postoperative chylous ascites following lymph node dissection, although rare in the field of gynecologic oncology, is still present despite the utilization of new technologies that improve intraoperative visualization and dexterity, such as the da Vinci robot. General lymph node dissection surgical principles, including careful clipping or coagulation of the lymphatic channels, may not suffice to prevent chylous ascites, especially in the cases where an enlarged lymph node is dissected.
Figures and Tables
References
1. Cardenas A, Chopra S. Chylous ascites. Am J Gastroenterol. 2002. 97:1896–1900.
2. Vettoretto N, Odeh M, Romessis M, Pettinato G, Taglietti L, Giovanetti M. Acute abdomen from chylous peritonitis: a surgical diagnosis. Case report and literature review. Eur Surg Res. 2008. 41:54–57.
3. Aalami OO, Allen DB, Organ CH Jr. Chylous ascites: a collective review. Surgery. 2000. 128:761–778.
4. Yen CF, Wang CJ, Lin SL, Lee CL, Soong YK. Postlaparoscopic vulvar edema, a rare complication. J Am Assoc Gynecol Laparosc. 2003. 10:123–126.
5. Kaas R, Rustman LD, Zoetmulder FA. Chylous ascites after oncological abdominal surgery: incidence and treatment. Eur J Surg Oncol. 2001. 27:187–189.
6. Shapiro AM, Bain VG, Sigalet DL, Kneteman NM. Rapid resolution of chylous ascites after liver transplantation using somatostatin analog and total parenteral nutrition. Transplantation. 1996. 61:1410–1411.
7. Giovannini I, Giuliante F, Chiarla C, Giordano M, Ardito F, Vellone M, et al. External lymphatic fistula after intra-abdominal lymphadenectomy for cancer: treatment with total parenteral nutrition and somatostatin. Nutrition. 2008. 24:1220–1223.
8. Collard JM, Laterre PF, Boemer F, Reynaert M, Ponlot R. Conservative treatment of postsurgical lymphatic leaks with somatostatin-14. Chest. 2000. 117:902–905.
9. Weinstein LD, Scanlon GT, Hersh T. Chylous ascites: management with medium-chain triglycerides and exacerbation by lymphangiography. Am J Dig Dis. 1969. 14:500–509.
10. Molina WR, Desai MM, Gill IS. Laparoscopic management of chylous ascites after donor nephrectomy. J Urol. 2003. 170:1938.
11. Castillo OA, Litvak JP, Kerkebe M, Olivares R, Urena R. Case report: laparoscopic management of massive chylous ascites after salvage laparoscopic retroperitoneal lymph-node dissection. J Endourol. 2006. 20:394–396.
12. Zeidan S, Delarue A, Rome A, Roquelaure B. Fibrin glue application in the management of refractory chylous ascites in children. J Pediatr Gastroenterol Nutr. 2008. 46:478–481.
13. Ablan CJ, Littooy FN, Freeark RJ. Postoperative chylous ascites: diagnosis and treatment. A series report and literature review. Arch Surg. 1990. 125:270–273.
14. Ryan JA Jr, Smith MD, Page CP. Treatment of chylous ascites with peritoneo-venous shunt. Am Surg. 1981. 47:384–386.
15. Le Pimpec-Barthes F, Pham M, Jouan J, Bel A, Fabiani JN, Riquet M. Peritoneoatrial shunting for intractable chylous ascites complicating thoracic duct ligation. Ann Thorac Surg. 2009. 87:1601–1603.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download