Abstract
A 61-year old woman underwent total abdominal hysterectomy and pelvic lymph node dissection under the diagnosis of endometrial cancer. Although pelvic lymph nodes were positive for adenocarcinoma with psamomma bodies, no other lesion that was a primary lesion was verified. A postoperative study revealed the existence of para-aortic lymph node and supraclavicular lymph node metastases. Therefore, the endometrial biopsy specimen was reviewed. With the findings of p53 positivity by immunohistochemistry in the papillary part, the final histopathological diagnosis was changed to endometrial serous adenocarcinoma. Postoperative chemotherapy followed by radiotherapy for supraclavicular lymph node metastasis achieved complete response. This type of tumor must be considered in a differential diagnosis when metastatic papillary serous carcinoma is detected, but the primary site remains unknown.
Uterine serous adenocarcinoma (USC) accounts for less than 10% of endometrial cancers and is a highly aggressive tumor which often presents with extra-uterine spread [1]. Although recent studies show that serous endometrial intraepithelial carcinoma (EIC) is the precursor of USC, serous EIC demonstrates a stage-dependent potential for aggressive behavior similar to that of USC [2,3]. Therefore, surgical staging is recommended in the case of a USC, even though the intrauterine lesion is minimal. Wheeler et al. [2] proposed to define superficial serous carcinoma (SSC) as USC without myometrial and lymphovascular invasion, and serous EIC as minimal uterine serous carcinoma (minimal USC) because it is difficult to distinguish these two lesions based on identification of stromal invasion and clinical behavior. Among patterns of extra-uterine spread presented in 33% to 45% of minimal USC [2,3], distant lymph node metastasis has not been previously reported. Therefore, we describe a case with minimal USC showing an unusual spread pattern, with cervical lymph node metastasis through the pelvic and para-aortic lymph nodes.
A 61-year old woman (gravida 2, para 2) presented at the obstetrics and gynecology clinic with postmenopausal vaginal bleeding. She was referred to our department because of positive endometrial cytology. Pelvic examination results were poor due to obesity, with a body mass index of 35.0 kg/m2. Ultrasonography and magnetic resonance imaging of the pelvic organs revealed not increased endometrial thickness but rather, a small myoma. Endometrial curettage revealed a grade 1 endometrioid adenocarcinoma from the villoglandular proliferation of atypical cells (Fig. 1A). Colonoscopy and cystoscopy showed abnormal findings on the surface of the rectum and bladder, respectively. Chest and abdominal computed tomography revealed no abnormality including the pelvic and para-aortic lymph nodes. Serum CA-125 levels were elevated to 41.8 U/mL.
Laparotomy was performed under the diagnosis of endometrial cancer. No macroscopic abnormalities were seen in the abdominal cavity, including the pelvic and para-aortic lymph nodes. Total abdominal hysterectomy, bilateral salpingo-oophorectomy and biopsy of the external iliac lymph node was performed. Since the preoperative histopathological diagnosis was grade 1 endometrioid adenocarcinoma, and laparotomy revealed no swollen pelvic or para-aortic lymph nodes, dissection of these nodes was omitted. The endometrium was macroscopically atrophic in the extirpated uterus.
Histopathological examination revealed no lesions in the uterus, but adenocarcinoma was present accompanied by psammoma body in the external iliac lymph node (Fig. 1B). Peritoneal washing cytology was negative. Based on pre- and postoperative histopathological findings, the final diagnosis was endometrial carcinoma stage IIIC (pT1aN1M0) according to the International Federation of Obstetrics and Gynecology (FIGO) classification. After surgery, 18-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) revealed left supraclavicular and para-aortic lymph node metastases. Therefore, we reviewed the specimen of the endometrial biopsy. The papillary part with prominent nuclear atypia in the villoglandular architecture was observed in the specimen (Fig. 1C). Immunohistochemical staining indicated the expression of p53 both in the papillary part of the biopsied endometrium (Fig. 2A) and the metastasized pelvic lymph node (Fig. 2B). Expression of estrogen receptor, progesterone receptor, and PTEN was not observed immunohistochemically (data not shown). Thus, the final histopathological diagnosis was an endometrial serous adenocarcinoma together with a metastatic lymph node.
This patient underwent 6 cycles of chemotherapy with a combination of cisplatin (50 mg/m2) and doxorubicin (60 mg/m2). Additional external beam radiation therapy (Liniac 3 Gy ×15) was given to the left supraclavicular region because the left supraclavicular lymph node remained swollen after chemotherapy. The patient maintained no evidence of disease 37 months after operation.
USC is uncommon, but accounts for a disproportionate number of endometrial cancer deaths. Hamilton et al. [4] reported that the poorer prognosis of USC and clear cell carcinoma might be due to advanced status at presentation, although both remain as independent prognostic factors for poor survival in multivariate analysis. USC is a highly aggressive carcinoma and shows different spread patterns from endometrioid adeno-carcinoma. Deep myometrial invasion is the strongest predictor of extrauterine spread of disease in endometrioid adenocarcinoma. On the other hand, the absence of myometrial invasion does not predict the absence of lymph node involvement or extrauterine metastasis in uterine papillary serous carcinoma [5]. Several studies revealed that 50% to 75% of clinical stage I USC was upstaged by surgical staging [6-8]. Even though the primary lesion in the endometrium was minimal, 33% to 45% of USC spread outside the uterus [2,3]. In the present case, pelvic and para-aortic lymph node metastasis was observed without peritoneal dissemination. Furthermore, supraclavicular lymph node metastasis was detected by FDG-PET. This may be via the regional lymph nodes. These reports indicate that surgical staging is recommended even in cases of non-invasive endometrial cancer when serous adenocarcinoma is suspected. In our case, complete surgical staging was not undertaken because USC was not diagnosed preoperatively and no significant malignant lesion was identified macroscopically at surgery. Since USC mimics low grade endometrioid adenocarcinoma, USC is sometimes difficult to differentiate from low grade endometrioid adenocarcinoma histopathologically [9]. Wheeler et al. [2] reported that 5 of 21 cases of minimal USC were originally diagnosed as endometrioid adenocarcinoma. Most of them were reclassified as USC from their aggressive clinical behavior despite the minimal volume of the lesion in the uterus. Thus, most cases initially classified as endometrioid adenocarcinoma showed well-formed gland like structure and marked nuclear atypia. Furthermore, they mentioned that the histo-pathological diagnosis of EIC and SSC may be challenging, especially in biopsies or curettages, because the lesions may be focal and small. Furthermore, recognizing the high nuclear grade in scant specimens, or suboptimally preserved material, can be problematic, leading to misdiagnosis. It was difficult to diagnose USC in our case for the same reasons described above. Immunohistochemical staining of p53 has been reported to be helpful in the diagnosis of USC [10], especially in distinguishing USC from architecturally well-differentiated tumors [9]. Regarding our case, the immunostaining of p53 was helpful in the diagnosis of minimal USC as well.
In conclusion, we experienced a case of minimal USC, with regional and distant lymph node metastases. It also showed an unusual spread pattern with distant metastasis despite a lack of intraperitoneal dissemination. USC must be suspected when encountering cases with extrauterine spread of disease, regardless of tumor size. Although it is challenging to make a diagnosis with curetted out specimen, with small fraction, tumor spreading pattern and immunohistochemical findings were helpful in making the final diagnosis.
Figures and Tables
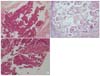
Fig. 1
(A) The glandular pattern of proliferation in a small fragment was observed (H&E, ×100). (B) Metastasized lymph node with adenocarcinoma accompanied by psamomma bodies (H&E, ×200). (C) The papillary part with prominent nuclear atypia in the villo-glandular architecture was observed in the specimens obtained from D&C (H&E, ×200).
ACKNOWLEDGEMENTS
A part of this work was supported by the Supporting Fund of Obstetrics and Gynecology, Kurume University. We thank Dr. Masayoshi Kage and C.T. Tomohiko Yamaguchi for help with immunostaining and pathohistological diagnosis.
References
1. Abeler VM, Kjorstad KE. Serous papillary carcinoma of the endometrium: a histopathological study of 22 cases. Gynecol Oncol. 1990. 39:266–271.
2. Wheeler DT, Bell KA, Kurman RJ, Sherman ME. Minimal uterine serous carcinoma: diagnosis and clinicopathologic correlation. Am J Surg Pathol. 2000. 24:797–806.
3. Hui P, Kelly M, O'Malley DM, Tavassoli F, Schwartz PE. Minimal uterine serous carcinoma: a clinicopathological study of 40 cases. Mod Pathol. 2005. 18:75–82.
4. Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006. 94:642–646.
5. Acharya S, Hensley ML, Montag AC, Fleming GF. Rare uterine cancers. Lancet Oncol. 2005. 6:961–971.
6. Grice J, Ek M, Greer B, Koh WJ, Muntz HG, Cain J, et al. Uterine papillary serous carcinoma: evaluation of long-term survival in surgically staged patients. Gynecol Oncol. 1998. 69:69–73.
7. Goff BA, Kato D, Schmidt RA, Ek M, Ferry JA, Muntz HG, et al. Uterine papillary serous carcinoma: patterns of metastatic spread. Gynecol Oncol. 1994. 54:264–268.
8. Christman JE, Kapp DS, Hendrickson MR, Howes AE, Ballon SC. Therapeutic approaches to uterine papillary serous carcinoma: a preliminary report. Gynecol Oncol. 1987. 26:228–235.
9. Darvishian F, Hummer AJ, Thaler HT, Bhargava R, Linkov I, Asher M, et al. Serous endometrial cancers that mimic endometrioid adenocarcinomas: a clinicopathologic and immunohistochemical study of a group of problematic cases. Am J Surg Pathol. 2004. 28:1568–1578.
10. Zheng W, Khurana R, Farahmand S, Wang Y, Zhang ZF, Felix JC. p53 immunostaining as a significant adjunct diagnostic method for uterine surface carcinoma: precursor of uterine papillary serous carcinoma. Am J Surg Pathol. 1998. 22:1463–1473.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download