Abstract
Figures and Tables
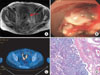 | Fig. 1(A) Magnetic resonance imaging showing the metastatic choriocarcinoma involving the rectosigmoid colon. (B) Sigmoidoscopy display a lobulating mass which was confirmed as choriocarcinoma. (C) Positron emission tomography scan showing increased fludeoxyglucose (FDG) uptake in the rectosigmoid colon which is consistent with malignant tissue. Choriocarcinoma with syncytiotrophoblastic and cytotrophoblastic elements which metastasized to the colon. (D) There is extensive inflammatory response with no chorionic villi. The left side is the mucosal aspect, and the right side is the serosal aspect (H&E, ×200). |
Table 1

hCG, human chorionic gonadotropin; NA, not available; MTX-CF, methotrexate and folic acid rescue; TAH, total abdominal hysterectomy; BSO, bilateral salpingo-oophorectomy; LAR, low anterior resection; NED, no evidence of disease.
*MTX-CF, methotrexate 1.0 mg/kg+CF 0.1 mg/kg. †Etoposide 100 mg/m2+methotrexate 300 mg/m2+actinomycin D 0.5 mg+ cyclophosphamide 600 mg/m2+vincristine 1 mg/m2+CF 15 mg.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download