Abstract
Objective
Syndecans are reported to have variable expression in several solid tumors and blood cancers. The cause provoking altered expression of syndecans is not known to date. We studied copy number status of syndecan-1 (SDC1) and significance of SDC1 gene product (syndecan-1, SDC1) expression in cervical cancers.
Methods
Using 121 cases of cervical cancer tissues, we screened SDC1 expression pattern using immunohistochemistry. We analyzed the relationship between SDC1 expression and clinicopathological parameters. To find possible causes of the expression change, we exploited interphase fluorescent in situ hybridization to screen copy number alteration of SDC1.
Results
Among 121 cases, 101 (83.5%) were positive and 20 (16.4%) were negative for SDC1. Among the parameters, age, histological type, and grade were significantly associated with SDC1 expression (p<0.05). Strong SDC1 expression in the cytoplasm showed better patient survival (p=0.02). In multivariate regression model, grade and SDC1 expression were independent prognostic factors (p<0.05). SDC1 in cervical cancers did not show copy number alteration.
With the help of effective screening and treatment methods, mortality of cervical cancer has been greatly reduced. However, cervical cancer still is the second leading cause of cancer deaths in women worldwide [1]. The single most important factor in cervical oncogenesis is high oncogenic risk human papillomaviruses (HPVs). HPVs interfere with Rb and p53 function to make cervical epithelial cells cancer-prone state. However, only a small portion of HPV-infected patients develop cervical cancer and this necessitates further studies of other factors, including cancer-related genes at the molecular level. Among the genes studied so far, fibroblast growth factor receptor (FGFR) is found constitutively activated [2]. Since the introduction of genome-wide, high-throughput research modalities, many studies to find disease-specific markers were conducted. As a result, they proposed several genes or gene products to have prognostic significance. Among the targets, significance of syndecans is reported in a few solid tumors and blood cancers.
Syndecans are plasma membrane proteoglycans and their cytoplasmic domain is thought to interact with the actin cytoskeleton [3]. They act as co-receptors by binding fibroblast growth factors (FGFs) and presenting them to FGFRs [3]. There are 4 different kinds of syndecans in vertebrates [4]. Among syndecans, which are expressed in various types of cells, syndecan-1 (SDC1) is expressed in epithelia and plasma cells [5].
Several studies on the relationship between syndecan expression and cancers were conducted. SDC1 expressions in various tumors, including head and neck cancer [6], hepatocellular carcinoma [7], mesothelioma [8], and lung cancer [9], were decreased. However, in some malignant tumors, such as endometrial cancer [10], ovarian cancer [11] and pancreatic cancer [12], SDC1 expression is increased. Those studies reported quantitative changes of SDC1 expression and the nature of the changes is dependent on the organ that the tumor has occurred. The syndecan family of matrix receptors is known to involve integrin (β4)-dependent signaling in human squamous carcinoma cells [13].
Prognostic significance of SDC1 expression is evaluated in several human cancers. Gallbladder cancer with SDC1 expression showed more frequent lymph node metastasis [14].
In this study, we compared SDC1 expression with several pathologic parameters in cervical cancers to find any significant association. To find possible cause of changes in SDC1 expression, we measured copy numbers of SDC1 in cervical cancers using fluorescent in situ hybridization (FISH).
This study included tumor tissues surgically resected from 121 patients who visited Seoul St. Mary's Hospital and were diagnosed with uterine cervical cancer between 1999 and 2003. Patients' age was between 28 and 77 (mean, 50) years old. Most of patients were in FIGO stage I (69 cases, 57.0%) and II (48 cases, 39.7%). Eighty-two cases (68%) of patients were treated with combination chemotherapy consisting of cisplatin and etoposide before operation. Tumor-specific survival data (median, 61 months; range, 0.5 to 151 months) was available. The disease relapsed in 17 patients (14%), and 20 patients (17%) died of the disease. Using the tissues, tissue microarray (TMA) blocks were constructed and used for immunohistochemical staining and interphase FISH. Human tissue acquisition and its use followed the Institutional Review Board-approved protocol (CUMC10U917) at the Catholic University of Korea School of Medicine.
Sections from TMA blocks were transferred to ProbeOn Plus slides (Fisher Scientific, Pittsburgh, PA, USA) and incubated for two hours in 56℃ chamber (Agilent Technologies, Santa Clara, CA, USA). The sections were deparaffinized in xylene 3 times and rehydrated through 100%, 90%, 80%, 70% ethanol and Tris-buffered saline (TBS, pH 7.4). For antigen retrieval, the tissues were immersed in 10 mM sodium citrate buffer (pH 6.0) and boiled in a microwave for 20 minutes. After treating the tissues with 3% H2O2 in phosphate buffered saline (PBS), the tissues were incubated with diluted (1:50) mouse monoclonal antibody to SDC1 (Abcam, Cambridge, UK) at 4℃ overnight. After incubating the tissue with biotinylated anti-mouse antibody (Abnova, Walnut, CA, USA), TSA HRP System (PerkinElmer, Waltham, MA, USA) was used to amplify signal intensity. For visualization, liquid DAB+substrate chromogen system (Dako, Glostrup, Denmark) was used. Immunoreactivity of SDC1 was classified according to the percentage of tumor cells showing cytoplasmic stain; strong, >50% of cells stained; weak, 10-50% of cells stained; negative, <10% of cells stained. Two pathologists analyzed the immunoreactivity independently.
To synthesize SDC1 FISH probe, we used BioPrime Array CGH Genomic Labeling Module (Invitrogen, Carlsbad, CA, USA). BAC clone (PRP11-202B22; Invitrogen) was used as template and Spectrum Orange-dUTP (Abbott Molecular, Abbott Park, IL, USA) was used to label the probe. Aquarius Satellite probe of chromosome 2 (Cytocell, Cambridge, UK) was purchased as the reference probe. Location of homemade SDC1 probe was confirmed in metaphase spread of normal peripheral mononuclear cells. Tissue processing and hybridization was done using Paraffin Pretreatment Kit I (Abbott Molecular) and ThermoBrite (Abbott Molecular). We followed the manufacturer's recommended FISH protocols. We counted the number of fluorescent spots in at least 100 nuclei in each case.
Chi-square test and Fisher-exact test was used to evaluate significance of SDC1 expression in terms of pathologic features. The numbers of target spots and reference spots in FISH were compared using the chi-square test. For survival analysis, Kaplan-Meier method was used. To test the difference between survival curves of different groups, we used the nonparametric log-rank test. We used Cox's multivariate proportional hazard model to determine the prognostic values of selected clinicopathologic parameters. We used R ver. 2.10 (R foundation, Vienna, Austria) for statistical calculations and production of graphs.
Normal squamous epithelial cells showed membranous pattern of SDC1 expression. Cytoplasm of normal cervical epithelial cells did not show SDC1 expression. On the contrary, cytoplasmic pattern of SDC1 expression was found in cancer cells in 101 cases (83.5%) of cervical cancers (Fig. 1). We compared the status of cytoplasmic SDC1 expression and some pathologic parameters. SDC1 was more frequently expressed in squamous cell carcinomas than adenocarcinomas and adenosquamous carcinomas (p=0.001). High grade tumors had less percentage of SDC1 expression than low grade tumors (p=0.036). We could not find any correlation between status of recurrence, lymph node metastasis, tumor stage and SDC1 expression (Table 1). Multivariate analysis showed that tumor grade and strong SDC1 expression were independent prognostic factors for survival (Table 2).
There was no significant difference between the numbers of target (SDC1) spots and references spots (p>0.05) in the cases showing increased SDC1 immunoreactivity (Fig. 2).
We analyzed patient survival among the groups with different SDC1 expression. Difference between the group of strong SDC1 expression and other groups was statistically significant (p=0.0219) (Fig. 3).
In this study, we showed that SDC1 expression is increased in cervical cancer and that most of the SDC1 accumulates in the cytoplasm of cancer cells. The finding that high grade tumors show decreased expression of SDC1 is consistent with a previous report [15].
Silencing SDC1 expression causes reduced focal adhesion plaque formation and enhanced cell spreading and motility on collagen I substrates [16]. Considering the fact that spreading and motility of cancer cells are highly related to invasion and distant metastasis, SDC1 positive tumors might have a more favorable biological behavior. This possible benefit of SDC1 may explain the findings that SDC1 expression is more common in low grade tumors and that patients with strong SDC1 expression have more favorable outcome in survival analysis.
We also showed that increased amounts of SDC1 is confined to the cytoplasm of tumor cells. Normal membranous distribution of SDC1 vanished in the neoplastic cells. Due to transposition of SDC1 from the cytoplasmic membrane to cytoplasm, the cells lose effective SDC1 on their surfaces. Considering the function of SDC1, these cells might lose connection to the extracellular matrix and move more freely than normal cells. Increased mobility of the cells can contribute to invasion and metastasis. Alteration of SDC1 distribution may be caused by degradation of the glycosaminoglycan chains, or expression of a mutated core protein that cannot undergo glycanation [17].
In one report, an inverse correlation between the expression of SDC1 in the tumor and lymph node metastasis was suggested [18]. However, they failed to show statistical significance, as we did.
In other malignancies, such as endometrial cancers [19] and invasive ductal breast carcinomas [20], SDC1 expression is reported to be associated with better prognosis. However certain malignancies, such as gallbladder cancer [14] and prostate cancer [21], it has been reported to have shorter survival time with positive SDC1 expression. This contradictory effect of SDC1 might be due to the difference of the site of origin that the tumors have come from.
SDC1 can be detached from the cytoplasmic membrane and become soluble form. The soluble SDC1 is associated with shorter survival in chronic lymphocytic leukemia [22] and lung cancer [23]. SDC1 expression of the bone marrow environment is associated with shorter event-free survival of multiple myeloma [24]. Adverse effect of SDC1 expression in tumor stroma has also been reported in oral carcinomas [25] and pancreatic cancer [26].
Decreased expression of SDC1 can be ascribed to increased degradation of the protein in cancer cells without any change in the gene expression. As a mechanism of decreased SDC1, decreased biosynthesis of the protein is suggested in gastric cancer [27]. Sometimes changes in copy number of a gene result in alterations in gene expression. To find a cause of SDC1 expression change, we screened the copy number of SDC1 and found no alteration in its copy number. It seems that alteration of SDC1 expression is caused by changes other than copy number alteration. The mechanism of SDC1 expression in malignant cell is not known to date.
We found two reports claiming that SDC1 expression did not predict survival [28,29]. In those articles, authors scored immunoreactivity of SDC1 on the surface of cells. SDC1 can be found on the surface of normal epithelial cells, and cervical cancer cells may retain SDC1 expression on the surface. Because we focused on abnormal location of SDC1 in tumor cells, we scored cytoplasmic expression of SDC1. With this scoring scheme, we found that strong SDC1 expression was associated with better survival. This explains the difference of our results and previous reports.
In conclusion, we showed intense SDC1 expression is associated with better prognosis in cervical cancer. Tumor grade and histological type is also related to the SDC1 expression. The alteration of SDC1 expression in cervical cancer is not caused by copy number changes of SDC1 in the tumor.
Figures and Tables
Fig. 1
Representative syndecan-1 immunohistochemical staining in (A) normal cervical epithelium and cervical cancer, (B) negative, (C) weak positive, (D) strong positive (×200).
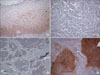
Fig. 2
Flurorescence in situ hybridization (FISH) of syndecan-1 (SDC1). (A) Location of homemade SDC1 probe was confirmed on metaphase spread of normal peripheral mononuclear cells. (B) There is no copy number alteration of SDC1 in interphase FISH of cervical cancer tissue (Red spot, SDC1; Green spot, chromosome 2 centromere).

Fig. 3
Survival analysis between groups showing different amount of syndecan-1 expression. Weak positive group and negative group have similar survival curve. Statistically significant difference was noted between strong positive group and other groups (p=0.0219).

ACKNOWLEDGMENTS
The authors wish to acknowledge the financial support of the Catholic Medical Center Research Foundation made in the program year of 2009.
References
1. Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010. 10:550–560.
2. Cappellen D, De Oliveira C, Ricol D, de Medina S, Bourdin J, Sastre-Garau X, et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999. 23:18–20.
3. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular biology of the cell. 2008. 5th ed. New York: Garland Science.
4. Rapraeger AC. Molecular interactions of syndecans during development. Semin Cell Dev Biol. 2001. 12:107–116.
5. Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999. 68:729–777.
6. Inki P, Jalkanen M. The role of syndecan-1 in malignancies. Ann Med. 1996. 28:63–67.
7. Matsumoto A, Ono M, Fujimoto Y, Gallo RL, Bernfield M, Kohgo Y. Reduced expression of syndecan-1 in human hepatocellular carcinoma with high metastatic potential. Int J Cancer. 1997. 74:482–491.
8. Kumar-Singh S, Jacobs W, Dhaene K, Weyn B, Bogers J, Weyler J, et al. Syndecan-1 expression in malignant mesothelioma: correlation with cell differentiation, WT1 expression, and clinical outcome. J Pathol. 1998. 186:300–305.
9. Nackaerts K, Verbeken E, Deneffe G, Vanderschueren B, Demedts M, David G. Heparan sulfate proteoglycan expression in human lung-cancer cells. Int J Cancer. 1997. 74:335–345.
10. Oh JH, Kim JH, Ahn HJ, Yoon JH, Yoo SC, Choi DS, et al. Syndecan-1 enhances the endometrial cancer invasion by modulating matrix metalloproteinase-9 expression through nuclear factor kappaB. Gynecol Oncol. 2009. 114:509–515.
11. Davies EJ, Blackhall FH, Shanks JH, David G, McGown AT, Swindell R, et al. Distribution and clinical significance of heparan sulfate proteoglycans in ovarian cancer. Clin Cancer Res. 2004. 10:5178–5186.
12. Conejo JR, Kleeff J, Koliopanos A, Matsuda K, Zhu ZW, Goecke H, et al. Syndecan-1 expression is up-regulated in pancreatic but not in other gastrointestinal cancers. Int J Cancer. 2000. 88:12–20.
13. Wang H, Leavitt L, Ramaswamy R, Rapraeger AC. Interaction of syndecan and alpha6beta4 integrin cytoplasmic domains: regulation of ErbB2-mediated integrin activation. J Biol Chem. 2010. 285:13569–13579.
14. Roh YH, Kim YH, Choi HJ, Lee KE, Roh MS. Syndecan-1 expression in gallbladder cancer and its prognostic significance. Eur Surg Res. 2008. 41:245–250.
15. Inki P, Stenback F, Grenman S, Jalkanen M. Immunohistochemical localization of syndecan-1 in normal and pathological human uterine cervix. J Pathol. 1994. 172:349–355.
16. Ishikawa T, Kramer RH. Sdc1 negatively modulates carcinoma cell motility and invasion. Exp Cell Res. 2010. 316:951–965.
17. Couchman JR. Syndecans: proteoglycan regulators of cell-surface microdomains? Nat Rev Mol Cell Biol. 2003. 4:926–937.
18. Numa F, Hirabayashi K, Kawasaki K, Sakaguchi Y, Sugino N, Suehiro Y, et al. Syndecan-1 expression in cancer of the uterine cervix: association with lymph node metastasis. Int J Oncol. 2002. 20:39–43.
19. Hasengaowa , Kodama J, Kusumoto T, Shinyo Y, Seki N, Hiramatsu Y. Prognostic significance of syndecan-1 expression in human endometrial cancer. Ann Oncol. 2005. 16:1109–1115.
20. Loussouarn D, Campion L, Sagan C, Frenel JS, Dravet F, Classe JM, et al. Prognostic impact of syndecan-1 expression in invasive ductal breast carcinomas. Br J Cancer. 2008. 98:1993–1998.
21. Zellweger T, Ninck C, Mirlacher M, Annefeld M, Glass AG, Gasser TC, et al. Tissue microarray analysis reveals prognostic significance of syndecan-1 expression in prostate cancer. Prostate. 2003. 55:20–29.
22. Jilani I, Wei C, Bekele BN, Zhang ZJ, Keating M, Wierda W, et al. Soluble syndecan-1 (sCD138) as a prognostic factor independent of mutation status in patients with chronic lymphocytic leukemia. Int J Lab Hematol. 2009. 31:97–105.
23. Joensuu H, Anttonen A, Eriksson M, Makitaro R, Alfthan H, Kinnula V, et al. Soluble syndecan-1 and serum basic fibroblast growth factor are new prognostic factors in lung cancer. Cancer Res. 2002. 62:5210–5217.
24. Mahtouk K, Hose D, Raynaud P, Hundemer M, Jourdan M, Jourdan E, et al. Heparanase influences expression and shedding of syndecan-1, and its expression by the bone marrow environment is a bad prognostic factor in multiple myeloma. Blood. 2007. 109:4914–4923.
25. Mathe M, Suba Z, Nemeth Z, Tatrai P, Fule T, Borgulya G, et al. Stromal syndecan-1 expression is an adverse prognostic factor in oral carcinomas. Oral Oncol. 2006. 42:493–500.
26. Juuti A, Nordling S, Lundin J, Louhimo J, Haglund C. Syndecan-1 expression: a novel prognostic marker in pancreatic cancer. Oncology. 2005. 68:97–106.
27. Watari J, Saitoh Y, Fujiya M, Shibata N, Tanabe H, Inaba Y, et al. Reduction of syndecan-1 expression in differentiated type early gastric cancer and background mucosa with gastric cellular phenotype. J Gastroenterol. 2004. 39:104–112.
28. Rintala M, Inki P, Klemi P, Jalkanen M, Grenman S. Association of syndecan-1 with tumor grade and histology in primary invasive cervical carcinoma. Gynecol Oncol. 1999. 75:372–378.
29. Shinyo Y, Kodama J, Hasengaowa , Kusumoto T, Hiramatsu Y. Loss of cell-surface heparan sulfate expression in both cervical intraepithelial neoplasm and invasive cervical cancer. Gynecol Oncol. 2005. 96:776–783.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download