Abstract
Anastomotic leakage is a very significant complication after posterior pelvic exenteration and a major cause of postoperative morbidity and mortality. We present a patient who underwent an optimal debulking surgery for an advanced stage ovarian cancer (FIGO IIIC). On postoperative day 12, transvaginal ultrasound revealed an anastomotic dehiscence following an unsuspicious computer tomography scan the day before. The patient was successfully managed by transanal vacuum therapy without re-laparotomy within a period of 4 weeks after diagnosis. We conclude that high-resolution transvaginal ultrasound is a crucial method in the management of complications after surgery and even allow diagnosing leakages of colorectal anastomosis. In selected cases characterized by a small leak size and a local peritonitis confined to the pelvis a transanal vacuum therapy may avoid both surgical re-intervention and creating a secondary diverting stoma.
Posterior pelvic exenteration (PPE) is a significant procedure to achieve complete tumor removal within the pelvis in the surgical treatment of primary advanced ovarian cancer or, less frequently, of recurrent disease. In most cases of this supralevator-type resection a low colorectal anastomosis, which is usually located 4 to 7 cm above the anocutan line, is possible because the infraperitoneal rectum is preserved from invasion by the pelvic mass. Current studies, including a review of literature, report a frequency of clinically significant anastomotic leakage in up to 10%, mostly less than 4% [1,2]. Anastomotic dehiscence is associated with an increased early and long term morbidity and mortality, mostly accompanied by a surgical re-intervention, a longer hospitalization, an impairment of anorectal function, and a delay of subsequent chemotherapy [1-5].
A 44-year old woman (gravida 2, para 1) underwent an optimal debulking surgery defined as complete tumor removal because of ovarian cancer FIGO stage IIIC characterized by a pronounced intraperitoneal tumor spread. Midline laparotomy includes the following procedures: posterior pelvic exenteration (PPE; en bloc resection of inner genitals, about 19 cm rectosigmoid and complete pelvic peritoneum), infragastric omentectomy, appendectomy, extensive deperitonealization of the right hemidiaphragm, removal of multiple peritoneal tumor deposits and, finally, a systematic pelvic and para-aortic lymph node dissection. A functioning colorectal anastomosis was realized by end-to-end circular stapling. The anastomotic doughnuts were complete and, additionally, the integrity of anastomosis was tested by insufflation of methylene blue-stained saline solution.
Histopathology revealed a serous adenocarcinoma originating from the ovaries (TNM-classification [2010]: pT3c, pN1 [5/97], pM1 LYM [1/19], L1, V0, G3).
The patient's postoperative recovery was uneventful over the next few days and her condition improved continuously. However, increased inflammation indicating values, leukocytes and C-reactive protein, decreased only gradually. After exclusion of possible foci a 'blind' antibiotic treatment with cefuroxim and metronidazol was initiated on postoperative day 5. Despite this therapy, the patient had complained of fever up to 38.5℃ every afternoon since the eighth day.
On day 11 after surgery, leukocytes and C-reactive protein increased considerably in comparison with the values of the day before (11.3 → 15.5×109/L; 146.2 → 207.3 mg/L). An abdominopelvic computer tomography scan revealed infected lymph cysts surrounding the iliac vessels on both sides (right, 7×6 cm; left, 8×7 cm), but there was no suspicion of any anastomotic leak. The antibiotic treatment was changed to imipenem. Regardless of the continued high temperature during the afternoon, the patient felt relatively comfortable; her bowel function was inconspicuous.
One day later, the causal diagnosis of an anastomotic dehiscence, located 5 cm above the anocutan line, was demonstrated by transvaginal ultrasound (Fig. 1A) and was verified by endoscopy. Considering the patient's condition, it was decided to attempt an Endo-SPONGE® assisted treatment (Fig. 1B; Aesculap AG, Tuttlingen, Germany). A parenteral nutrition for two weeks was initiated and during the nine-day transanal vacuum therapy the sponge was changed twice. Subsequently, a bowel rinsing was semi-weekly performed over the following two weeks (Fig. 1C-H). The anastomotic leakage healed successfully and the patient recovered uneventfully and was discharged on postoperative day 29. The first cycle of combination chemotherapy consisting of Caboplatin AUC 5 and Paclitaxel 175 mg/m2 could be applied two weeks later.
The application of transvaginal ultrasound to diagnose a leakage of colorectal anastomosis has not been previously described. The diagnostic criteria are the discontinuity of the rectal wall and an oscillating fluid flow between the bowel lumen and a surrounding hyperechogenic fluid collection, which is induced by pushing in the bowel wall by means of the ultrasound probe. In our opinion, ultrasound diagnostics should be integrated early in the diagnostic management of divergences from the expected course after any surgery.
Further points of interest are the use of protective stoma to prevent anastomotic leakage and the treatment options of this complication in patients receiving PPE for ovarian cancer. The authors of a multicenter study concluded that a temporary protective stoma should be considered only exceptionally, because the rates of "anastomotic fistula" were the same with and without stoma, respectively (8.5% vs. 8.2%) [1]. In addition, there are only a few published studies including small numbers of patients [3]. Our empiric intraoperative management is in accord with this recommendation. However, if an anastomotic dehiscence occurs, a re-operation to secondarily create a covering ileo- or colostomy or to drain the pelvic cavity in order to prevent severe septic complications is necessary in the majority of these cases [4]. The endoluminal vacuum therapy of anastomotic leakages has been increasingly applied following resections in both the upper and lower gastrointestinal tract over the last few years [4,5]. This approach can be integrated into both a surgical and conservative treatment regime, but the therapeutic effectiveness has not been verified by randomized clinical trials. Reports about the application of this method in the field of gynecologic oncology are absent.
Our case demonstrates, firstly, that anastomotic leakage of colorectal anastomosis can be obviously diagnosed by transvaginal ultrasound and, secondly, in selected cases characterized by a small leak size and a local peritonitis confined to the pelvis, an attempt of conservative management based on transanal vacuum therapy is justified and may avoid creating a secondary diverting stoma.
Figures and Tables
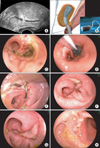 | Fig. 1(A) Transvaginal ultrasound of the rectum revealed a distinct anastomotic leak in the posterior wall (arrow). (B) Schematic drawing of the transanal Endo-SPONGE® insertion into an abscess cavity following examination and rinsing of the cavity using a flexible endoscope. Inset: The Endo-SPONGE® is an open-pored, cylindrical polyurethane sponge connected to a drainage tube which is linked to a vacuum system to exert constant suction (authorized by Aesculap AG, Germany). (C-H) Endoscopic images demonstrating the treatment course: initial finding of the anastomotic disruption with two openings of 15 and 3 mm (C), first-time insertion of the sponge fitted to the size of the pelvic abscess cavity measuring 30×30 mm (D), progressive obliteration of the cavity and narrowing of the opening by granulation tissue on days 9 (E), 13 (F) and 20 (G), and final finding of the anastomotic site almost completely covered by mucosa on day 27 (H) after initial diagnosis of the leakage. |
ACKNOWLEDGMENTS
The authors are very grateful to all nurses and physicians who are involved in the complex treatment of ovarian cancer patients at the University Hospital Leipzig.
References
1. Houvenaeghel G, Gutowski M, Buttarelli M, Cuisenier J, Narducci F, Dalle C, et al. Modified posterior pelvic exenteration for ovarian cancer. Int J Gynecol Cancer. 2009. 19:968–973.
2. Lim SW, Lim SB, Park JY, Park SY, Choi HS, Jeong SY. Outcomes of colorectal anastomoses during pelvic exenteration for gynaecological malignancy. Br J Surg. 2008. 95:770–773.
3. Tixier H, Fraisse J, Chauffert B, Mayer F, Causeret S, Loustalot C, et al. Evaluation of pelvic posterior exenteration in the management of advanced-stage ovarian cancer. Arch Gynecol Obstet. 2010. 281:505–510.
4. van Koperen PJ, van Berge Henegouwen MI, Rosman C, Bakker CM, Heres P, Slors JF, et al. The Dutch multicenter experience of the endo-sponge treatment for anastomotic leakage after colorectal surgery. Surg Endosc. 2009. 23:1379–1383.
5. Riss S, Stift A, Meier M, Haiden E, Grunberger T, Bergmann M. Endo-sponge assisted treatment of anastomotic leakage following colorectal surgery. Colorectal Dis. 2010. 12:e104–e108.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download