Abstract
Ovarian metastases from a primary urinary tract carcinoma are extremely rare. This can be difficult to distinguish from transitional cell carcinomas (TCC) of ovarian origin because of histologic similarity. A 65-year-old woman who was diagnosed with renal pelvis TCC 4 months prior was referred for evaluation of a left ovarian mass. A 47-year-old woman who underwent radical cystectomy due to bladder TCC 1 year ago was referred because of a right ovarian mass. Both patients underwent a bilateral salpingo-oophorectomy. The tumor cells had morphology identical to those of the primary urinary tract tumors. Gynecologic oncologists should consider metastatic TCC of the ovary from urinary tract origin, as well as breast, and gastrointestinal tract origins.
Ovarian metastases from a primary urinary tract carcinoma, especially those mimicking primary ovarian carcinoma, are very rare. The tumors that metastasize to the ovary commonly arise in the colorectum, breast, endometrium, stomach, cervix, pancreas, appendix.1 In one autopsy study, ovarian metastasis was found in 0.5% of cases of renal cell cancer.2 There are few reported cases of a primary transitional cell carcinoma (TCC) of the renal pelvis metastatic to the ovary.3 The purpose of this report is to present two cases of ovarian metastasis from TCC of urinary tract origin and to provide a brief review of the literature.
A 65-year-old woman with a left ovarian mass was referred to the gynecology department. She had received total abdominal hysterectomy due to a leiomyoma several years ago. She was diagnosed with TCC of the renal pelvis 4 months prior and underwent right nephroureterectomy with bladder cuff resection (Fig. 1A).
Postoperatively, she was seen for regular follow-up; her urologist obtained a computed tomography (CT) of her abdomen for surveillance. The CT revealed a 4.4×2.9 cm solid mass in the left adenexa, suggestive of an ovarian tumor. There was no evidence of abnormal lymphadenopathy, no obvious metastatic foci in other organs. Routine blood and biochemical test results were all within normal ranges. Before surgery, the initial investigation of tumor markers revealed a serum CA-125 of 4.8 U/mL, and other markers were all within normal ranges. She underwent bilateral salpingo-oophorectomy. At the time of surgery, a 4.5×4.5×4 cm left ovarian mass was noted with no evidence of any intraperitoneal or omental metastasis. The surface was smooth and glistening without perforation (Fig. 1B). The cut surface was yellowish-tan with multifocal, slightly whitish areas, and multiple fibrous septae were noted. Some small, cystic spaces containing clear, mucinous fluid were seen. The right ovary, fallopian tube and the cytology of peritoneal fluid was negative for malignant cells. The tumor cells were microscopically identical to those of the renal pelvis tumor (Fig. 1C, D).
After recovering from surgery, she received six cycles of chemotherapy with gemcitabine-carboplatin. The patient is presently doing well without any recurrent disease as of November, 2008.
A 47-year-old woman was referred to our institution because of a right ovarian mass that was noticed by her urologist during a routine CT of her abdomen. She had a past history of a radical cystectomy with neobladder 1 year prior for papillary TCC of the bladder, high grade, with lymph node metastasis. Postoperatively, she received only one cycle of chemotherapy with gemcitabine and cisplatin because of her poor general condition. She received regular follow-up and was asymptomatic. A CT imaging showed an 8×6.4×8.4 cm newly-developed mass in the right adnexa, suggestive of ovarian malignancy. Multiple borderline-sized para-aortic and aorto-caval lymph nodes were noted. Routine blood and biochemical test results were all within normal ranges. Before surgery, CA-19-9 was 116 U/mL, but other markers were all within normal ranges. She underwent a total abdominal hysterectomy with bilateral salpingo-oophorectomy and infracolic omentectomy. A 10×7×5 cm sized right ovarian mass was noted with no evidence of any intraperitoneal or omental metastasis. The ovary was cystically enlarged with a gray-white, smooth inner surface. Yellow-white solid areas were noted with spotty necrosis. The right ovary tumor cell was metastatic TCC. Immunohistochemical stains for cytokeratin 7, 13, 20 were positive. The left ovary and fallopian tube were negative for tumor.
She received three cycles of chemotherapy with gemcitabine after the surgery, and died 6 months later due to acute renal failure.
The ovaries are common sites for intra-abdominal metastasis. About 6% of ovarian cancers found at laparotomy are secondary tumors from other sites.4 In a register study, ovarian metastasis from a primary renal tumor was found to be 0.8 %.5 In the literature, only 14 cases have been reported. Of these, 13 cases were metastases of renal adenocarcinoma of clear cell type, and only one case was from a TCC of the renal pelvis.5 The most common renal tumor to metastasize to the ovary is a typical clear cell carcinoma. Metastatic TCC involving the ovary from the urinary bladder or elsewhere within the urinary is extremely rare.6 There have been six cases reported to date, as summarized in Table 1. In all cases, secondary ovarian tumors are unilateral. The time interval of appearance of ovarian metastases varied from synchronous to 4 years. All cases received surgery, with the overall survival ranging from 3 months to 7 years.
Microscopically, metastatic TCC of ovary is very similar to a primary ovarian TCC. Primary TCC accounts for 1% to 2% of all ovarian tumors.7 TCC of the ovary is a recently recognized subtype of ovarian surface epithelial-stromal cancer, and studies of its morphology are rare. The presence of a component of benign or borderline Brenner tumor confirms an ovarian primary. TCC of the ovaries has mucin pools, thick papillae with smooth luminal borders, in contrast to the pseudo-papillae of tumor cell necrosis that is common in metastatic TCC.8
The current cases favor metastatic ovarian tumors for the following reasons: Definite histological evidence of a primary renal tumor, and deep stromal invasion. The origin of primary lesions has prognostic significance because TCC of the ovary has a modest response to chemotherapy8 and metastatic TCC from the renal pelvis is universally fatal.
In a previous review, a delay in diagnosis of the primary lesion from the kidney occurred in 38% of patients with presenting symptoms corresponding to the metastatic site rather than the primary lesion.9 Limited evidence suggests that surgical extirpation of both lesions may lead to long-term disease-free survival.9 Thus, timely diagnosis of the primary lesions is critical.
To the best of our knowledge, this is the first report of case series of the TCC with primary renal pelvis, bladder tumor and ovarian metastasis. This report may help improve the current understanding of metastatic ovarian cancer.
Figures and Tables
Fig. 1
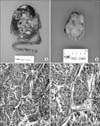
(A) Gross appearance of the right kidney and ureter showing TCC of the renal pelvis. (B) Gross appearance of the left ovary showing a solid rubbery mass that nearly replaces the ovarian parenchyma. (C) Microscopic finding of transitional cell carcinoma of the renal pelvis (H&E, ×100). (D) Metastatic transitional cell carcinoma of the ovary (H&E, ×200).
References
1. Demopoulos RI, Touger L, Dubin N. Secondary ovarian carcinoma: a clinical and pathological evaluation. Int J Gynecol Pathol. 1987. 6:166–175.
2. Young RH, Hart WR. Renal cell carcinoma metastatic to the ovary: a report of three cases emphasizing possible confusion with ovarian clear cell adenocarcinoma. Int J Gynecol Pathol. 1992. 11:96–104.
3. Hsiu JG, Kemp GM, Singer GA, Rawls WH, Siddiky MA. Transitional cell carcinoma of the renal pelvis with ovarian metastasis. Gynecol Oncol. 1991. 41:178–181.
4. Petru E, Pickel H, Heydarfadai M, Lahousen M, Haas J, Schaider H, et al. Nongenital cancers metastatic to the ovary. Gynecol Oncol. 1992. 44:83–86.
5. Skírnisdóttir I, Garmo H, Holmberg L. Non-genital tract metastases to the ovaries presented as ovarian tumors in Sweden 1990-2003: occurrence, origin and survival compared to ovarian cancer. Gynecol Oncol. 2007. 105:166–171.
6. Valappil SV, Toon PG, Anandaram PS. Ovarian metastasis from primary renal cell carcinoma: report of a case and review of literature. Gynecol Oncol. 2004. 94:846–849.
7. Young RH, Scully RE. Urothelial and ovarian carcinomas of identical cell types: problems in interpretation. A report of three cases and review of the literature. Int J Gynecol Pathol. 1988. 7:197–211.
8. Eichhorn JH, Young RH. Transitional cell carcinoma of the ovary: a morphologic study of 100 cases with emphasis on differential diagnosis. Am J Surg Pathol. 2004. 28:453–463.
9. Gavallos G, Tawfik O, Herrell D, Langenstroer P. Renal-ovarian axis: a case report and review. Urology. 2003. 62:749.
10. Raabe NK, Fossa SD, Bjerkehagen B. Carcinoma of the renal pelvis: experience of 80 cases. Scand J Urol Nephrol. 1992. 26:357–361.
11. Ishii Y, Itoh N, Takahashi A, Masumori N, Ikeda T, Tsukamoto T. Bladder cancer discovered by ovarian metastasis: cytokeratin expression is useful when making differential diagnosis. Int J Urol. 2005. 12:104–107.
12. Kardar AH, Lindstedt EM, Tulbah AM, Bazarbashi SN, al Suhaibani HS. Metastatic transitional cell carcinoma of the ovary from superficial bladder tumour. Scand J Urol Nephrol. 1998. 32:73–76.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download