Abstract
Objective
Regulatory T lymphocytes evoke the immune tolerance by suppressing and inactivating cytotoxic T lymphocytes. The objective of this study was to compare the proportion of regulatory T lymphocytes, precisely defined as CD4+CD25high+Foxp3+ T lymphocytes, in primary and recurrent ovarian carcinoma before and after ex vivo expansion of ascites with interleukin-2 (IL-2).
Methods
Ascitic fluid samples were obtained from 26 patients with ovarian carcinoma. Lymphocytes were isolated from ascites and cell markers were analyzed by flow cytometry using anti-CD3/CD4/CD8/CD16/CD56/CD25 and anti-Foxp3 antibodies. Lymphocytes were incubated for 2 to 3 weeks and expanded ex vivo by IL-2 stimulation and their phenotypes were analyzed by flow cytometry.
Results
Following ex vivo expansion, ascitic fluid lymphocytes increased by a greater extent in the recurrent group than in the primary group. The proportion of ex vivo-expanded lymphocytes changed as follows; CD4+ T lymphocytes increased, CD8+ T lymphocytes decreased, and the proportion of CD3-CD16+56+ NK cells was unchanged. The proportion of CD4+CD25high+Foxp3+ regulatory T lymphocytes in CD4+ T lymphocytes increased after ex vivo expansion in both groups, but to a greater degree in the recurrent group.
Conclusion
This study showed that regulatory T lymphocytes, neither cytotoxic T lymphocytes nor NK cells, were extensively increased after ex vivo expansion, especially in recurrent ovarian carcinoma. These results may provide information that helps to guide the future development of adoptive immunotherapy against ovarian carcinoma.
The emergence of tumor cells results from the deregulation of cell growth, as well as the failure to provoke a sufficient immunological response to tumor cells and the imbalance of immune system contributes to the growth and metastasis of tumor cells.1 Accordingly, many physicians are attempting to improve immunologic function and antitumor effects through immunotherapy independently of or in parallel with conventional surgery and chemotherapy. With increased use of immunotherapy, there is growing interest in the nature and characteristics of regulatory T lymphocytes in the peripheral blood and tumor tissue of patients.2 The term "regulatory T lymphocytes" usually refers to T lymphocytes that specifically express CD4 and CD25.3-5 If regulatory T lymphocytes become activated, they interfere with the immune system, and reduce its ability to effectively remove infective agents, such as viruses and bacteria, while also potentially beneficial suppressing the inflammatory response evoked by the infection.6 Regulatory T lymphocytes were also found to suppress immune response to tumors as well; significantly, their levels increase in the peripheral blood of patients with malignant tumors.7,8 CD4+ regulatory T lymphocytes are characterized by several markers, CD25 (interleukin [IL]-2 chain), cytotoxic T lymphocyte-related antigen 4 protein (CTLA-4), glucocorticoid-induced tumor necrosis factor receptor family-related gene (GITR) and forkhead box P3 (Foxp3).9 They serve an immunoregulatory function by secreting immunosuppressant cytokines, such as TGF-β and IL-10, or by directly suppressing the activation of T lymphocytes.10 The possibility of regulating immune tolerance was suggested by the observation that the transcription factor, Foxp3, which has a demonstrated role in autoimmune diseases, induces the conversion of inexperienced T lymphocytes into CD4+CD25+ regulatory T lymphocyte phenotypes.11,12 Tumor-associated peripheral blood regulatory T lymphocytes are known to contribute to the growth of tumor cells by expressing CTLA-4 (CD152), GITR and Foxp3.13 Previous studies showing that CD4+CD25+ regulatory T lymphocytes increase not only in the peripheral blood of patients with colon, breast, pancreatic, gastric, esophageal, and ovarian carcinomas, but also in the lymph nodes near malignant ascites and tumors, suggest that CD4+CD25+ regulatory T lymphocytes are involved in tumor cell growth as well as hematogenous, lymphocytic and peritoneal metastasis. Accordingly, the degree to which regulatory T lymphocytes are expressed is expected to influence the survival rate of cancer patients and serve as a prognostic indicator.14 Although a number of studies have reported on the CD4+CD25+ regulatory T lymphocyte-dependent expression, growth and metastasis of tumor cells in the peripheral blood of cancer patients, a clinically effective therapy against ovarian carcinoma has yet to be established.
This study investigated changes in the proportion of immune cells, especially regulatory T lymphocytes from the ascitic fluid of patients with ovarian carcinoma, upon ex vivo expansion of lymphocytes. Specifically, this study attempted to identify changes in the immune system that are associated with ovarian carcinoma progression. Further, by determining the differences in the changing properties of ex vivo expanded regulatory T lymphocytes between untreated patients with primary ovarian carcinoma and those with recurrent ovarian carcinoma, this study sought to establish clinical uses for antitumor immunotherapy.
Malignant ascites was collected prior to surgery from 26 ovarian carcinoma patients at the Asan Medical Center, Seoul, Korea, who were hospitalized between January 2005 and August 2006. We received informed consent and followed all guidelines for experimental investigation with human subjects required by the Institutional Review Board of the Asan Medical Center (Project No. 2007-0390). This study was performed in compliance with the Helsinki Declaration. Personal information, history and pathological findings were obtained from medical records. The collected ascites were centrifuged with serum free (SF)-RPMI (Invitrogen-Gibco, Grand Island, NY, USA) and 100% Histopaque (Sigma-Aldrich, Inc., St. Louis, MO, USA) at a ratio of 1 : 1. The 75% Histopaque solution was gently layered over 100% Histopaque, and cell fluid was then floated onto the 75% solution. After centrifuging, the tumor cell and lymphocyte could be collected separately and lymphocytes were placed in culture or frozen for later use.
We used a revised version of the expansion method reported by Freedman et al.15 Briefly, lymphocytes in RPMI/10% fetal bovine serum (FBS) were plated in 24-well plates added with 400 IU/mL of IL-2 and incubated at 37℃ in a 5% humidified CO2 incubator. As cell density increased, cells were transferred to fresh containers added with 400 IU/mL of IL-2 after measuring cell numbers and viability. The cell marker analysis was conducted within two to three weeks after culturing, depending on the proliferation state of the lymphocytes.
A cell marker analysis using FACS flow cytometry (Becton Dickinson, Franklin Lakes, NJ, USA) was conducted on the lymphocytes from 21 patients before and after culturing, excluding 5 patients whose cell count did not reach the minimum requirement for cell marker analysis. To minimize the influence of contaminating non-lymphocytes, we verified that the purity and recovery of the light scatter gate for lymphocytes was at least 85% before analysis. For CD4+ and CD8+ T lymphocytes and NK cells marker analysis, 20 µL each of fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated anti-CD3, anti-CD4, anti-CD8, and anti-CD16/56 antibodies (BD pharmingen, San Diego, CA, USA) was added to each tube and incubated. After incubating, the mixtures were centrifuged twice using cold PBS solution containing 2% FBS and 0.02% NaN3. Washed cells were fixed with 100 µL fix buffer and analyzed by flow cytometry. Marker analysis was performed on regulatory T lymphocytes by adding 20 µL each of FITC PE-conjugated anti-CD4 and anti-CD25 antibodies (BD pharmingen) and incubating. The cells were washed and incubated in permeabilization buffer and normal rat serum, thereafter, added with 20 µL of APC-conjugated anti-Foxp3 antibody (eBioscience, San Diego, CA, USA). Cells were centrifuged with permeabilization buffer containing fix buffer and analyzed by flow cytometry.
On the basis of the marker analysis results, CD4+CD25+ T lymphocytes with log values for the expression of CD25 >102 and <104 were classified as CD25low+, and those with log value >104 were classified as CD25high+. CD4+CD25high+ T lymphocytes that expressed Foxp3 were defined as regulatory T lymphocytes. SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA) was used for comparative analysis of the data. The Mann-Whitney U-test was used for statistical analysis and a p-value lower than 0.05 was considered statistically significant.
Of the 26 patients enrolled in the study, 19 were primary ovarian carcinoma patients who had not been previously treated, and 7 were recurrent ovarian carcinoma patients who had received cytoreductive surgery and antitumor chemotherapy. The median age of patients in the two groups was 57 and 58 years, respectively. They were all advanced serous carcinoma and treated with initial cytoreductive surgery. Malignant ascites was obtained during the operation in patients with primary ovarian carcinoma, and at the time of being diagnosed as recurrences in patients with recurrent ovarian carcinoma. The median number of chemotherapy sessions that the recurrent ovarian carcinoma patients had undergone was 1 (range, 1 to 3); they had been treated with paclitaxel combined with platinum as adjuvant chemotherapy in the initial treatment, and then, other drugs were used diversely after recurrence (Table 1).
Cell numbers measured at the time of cell marker analysis between two and three weeks after the initial culture increased 1.8-fold, averaged across both groups; the increase in recurrent ovarian carcinoma was ~2.5-fold, which was significantly higher than the ~1.2-fold increase in primary ovarian carcinoma (p<0.05). The proportion of CD4+ T lymphocytes increased significantly during culture, from 34±16% before incubation to 56±19% after expansion. In contrast, the proportion of CD8+ T lymphocytes decreased significantly from 30±8% to 18±10%. Although NK cells, which correspond to CD3-CD16+56+ lymphocytes, showed a decreasing trend after incubation, this difference was not significant (Fig. 1). There were no significant differences between primary and recurrent ovarian carcinoma in CD4+ and CD8+ T lymphocytes and NK cells with ex vivo incubation (Table 2 and Fig. 2).
In this study, the fraction of CD4+ T lymphocytes defined as CD4+CD25high+Foxp3+ regulatory T lymphocytes was very small. Thus, changes in regulatory lymphocytes were greatly dependent on increases or decreases in the total CD4+ T lymphocyte population, in other words, the change of total CD4+ T lymphocytes can mask that of CD4+CD25high+Foxp3+ regulatory T lymphocytes (Fig. 3). To overcome this relationship, we examined the proportion of regulatory T lymphocytes in 11 patients whose increase in CD4+ T lymphocytes was higher than the average 46% increase observed across all 26 subjects (Figs. 1 and 2). Among these, seven were from primary and four were from recurrent ovarian carcinoma patients. In this subset of patients, the percentage of CD4+CD25high+Foxp3+ regulatory T lymphocytes in the total CD4+ T lymphocyte population was more than doubled during culture, significantly increasing from 0.32±0.17% to 0.72±0.42% (Fig. 1). The increase in the proportion of CD4+CD25high+Foxp3+ regulatory T lymphocytes in recurrent ovarian carcinoma was 247±228%, which was significantly higher than the 16±89% increase observed in primary ovarian carcinoma, even though there is no significant difference of regulatory T lymphocytes percentages between primary and recurrent cases before ex vivo expansion (0.32±0.12 vs. 0.33±0.17) (Table 2 and Fig. 2).
The human body recognizes alien substances that it encounters as antigens, triggering an immune response to destroy them. Most CD4+ T lymphocytes involved in this process are classified as Type 1 and 2 T-helper cells, which trigger cell-mediated and antibody-mediated immune responses, respectively. Some immune responses, however, are suppressed by a subtype of CD4+ T lymphocytes termed regulatory T lymphocytes, which produce cytokines that block the activation or function of T lymphocytes. Defects in this class of cells have been reported to cause chronic infections, tumors, autoimmune diseases, and allergic responses.3-5
As the role of the immune system in regulating the generation and growth of tumor cells has become clearer, various immunotherapeutic approaches to suppress the generation and differentiation of tumors have been proposed.16 Experiments using animal models have shown that, when present at appropriate levels, cytotoxic T lymphocytes can clear a considerable quantity of tumor cells.1 The expression of MHC class I surface molecules and co-stimulatory signals are required to effectively present tumor antigens and stimulate a cytotoxic T lymphocyte response. Also necessary is the proliferation of CD3+CD4+ cells, which exert a cooperative effect on cytotoxic CD3+CD8+ T lymphocytes. The likelihood that these immune responses are suppressed or genetically diminished in many cancer patients frames current attempts to treat tumors by reactivating suppressed or otherwise insufficient immune responses. Immunotherapy against tumor cells can be divided into two types: 1) active immunotherapy, which involves activation of various immune cells that have antitumor effects, such as NK cells, activated giant cells, and cytotoxic T lymphocytes; and 2) passive immunotherapy, which involves direct injection of anti-tumor cell antibodies or immune cells capable of removing tumor cells.1,17 Complicating such approaches is the fact that tumor cells themselves are capable of suppressing immunity by generating immunity-lowering substances, such as TGF-β, IL-6, and IL-10.18 Ongoing studies aim to improve immunological competence against specific tumor cells; however, uncertainty about the mechanism by which the immune system responds to tumor cells places limits on the clinical application of these approaches. The immune system of cancer patients presents a paradox. On one hand is a type of biological ignorance, in which host immune cells fail to recognize tumor antigens; on the other hand is the state of immunosuppression, in which the expression and differentiation of T lymphocytes, which might otherwise attack cancer cells bearing specific tumor antigens, are lost. Because of this, tumor cells are not removed, but instead continue to grow and spread by metastasis.19 The functional degradation of T lymphocytes in cancer patients is caused by TCR-dependent apoptosis mediated by Fas-Fas ligand interactions, the production of reactive oxygen by myelomonocytes, or the secretion of hydrogen peroxide by giant macrophages.20
Since the early 1970s, when the idea that T lymphocytes could suppress the human immune system was first introduced, regulatory T lymphocytes have been known to induce immune tolerance against external antigens and autoantigens, and to maintain immune homeostasis by secreting various cytokines.21 Like other T lymphocytes, regulatory T lymphocytes have antigen-recognizing α, β TCRs that are activated by combining with specific MHC class II surface molecules or stimulation by B7 molecules, such as CD80 and CD86, of the antigen-presenting cells. Once activated, they acquire immunosuppressant and regulating capacity through cytotoxic T-lymphocyte antigen-4 (CTLA-4), which exerts its suppressant effect indirectly by stimulating production of the immunosuppressive cytokines, TGF-β and IL-10, or directly by suppressing the activation of T lymphocytes.5 The antigens recognized by these TCRs act to suppress autoimmunity by preventing the immune response of other T lymphocytes to self. Regulatory T lymphocytes are largely divided into type 1 regulatory T lymphocytes, T-helper-3 cells, CD4+CD25+ regulatory T lymphocytes, and regulatory T lymphocytes with CD8+CD25+CD28+, Qa-1-dependent CD8, CD4-CD8-, and TCRγδ phenotypes. The CD4+CD25+ regulatory T lymphocytes, which account for 5 to 10% of CD4+ regulatory T lymphocytes, mature in the thymus gland and suppress the activation of peripheral lymphocytes with the help of TGF-β and CTLA-4, as noted above, and also suppress the activation and differentiation of CD4+ and CD8+ regulatory T lymphocytes.22 Recently, CD4+CD25high+ regulatory T lymphocytes that express CD25 more strongly than other CD4+CD25+ regulatory T lymphocytes have been identified. Unlike "standard" CD4+CD25+ regulatory T lymphocytes, these regulate immunity independently of cytokines, and activate CD4+CD25+ regulatory T lymphocytes by expressing more IL-2 receptors. However, these differences are not sufficient to fully account for the functional distinctions between CD25+ and CD25high+ regulatory T lymphocytes, which have been suggested to cause a faster immune response to various antigens.23
Currently, the known markers of CD4+CD25+ regulatory T lymphocytes include CD25 (IL-2-chain), Foxp3, CTLA-4 (CD152) and GITR. The recently discovered Foxp3 is known to be specifically expressed in regulatory T lymphocytes; both Foxp3 mRNA and protein are expressed at high levels in CD4+CD25+ regulatory T lymphocytes and at low concentration in CD4+CD25- regulatory T lymphocytes activated by antigen stimulation.24 Foxp3 is a transcription factor that plays a role in the growth of CD4+CD25+ regulatory T lymphocytes, inducing the conversion of inexperienced T lymphocytes to CD4+CD25+ regulatory T lymphocyte phenotypes.12 It is also known to act as a switch for initiating or concluding the immune response of regulatory T lymphocytes to tumor antigens, suggesting a possible role in regulating immune tolerance. Thus, adoptive immunotherapy targeting Foxp3 to remove CD4+CD25+ regulatory T lymphocytes is being developed in an attempt to improve the immune response to tumor cells. The potential of strategies designed to eliminate CD4+CD25+ regulatory T lymphocytes is demonstrated by studies showing that injecting monoclonal anti-CD25 antibodies into the cells of leukemia, bone marrow cancer, and fibrosarcoma improved the rejection capacity against tumor cells.25 However, because tumor cells also display autoantigens and monoclonal anti-CD25 antibodies have the potential to increase autoimmune disease, a method to produce CD4+CD25+ regulatory T lymphocyte-attacking cells - lymphokine activated killer (LAK) cells and NK cells - using IL-2 or by injecting vaccine was devised.21 Accordingly, adoptive immunotherapy employing IL-2 or IL-2-amplified tumor infiltrating lymphocytes (TIL) in patients with malignant melanoma has been reported to achieve a response rate of 40%.26 However, the general complexity of the immune system and differences in the degree to which different tumor cells respond constrain the clinical application of this approach.27
There have been several studies showing that the immune system is suppressed by increased numbers of regulatory T lymphocytes in patients with ovarian carcinoma. The experimental approach to expand adult lymphocytes ex vivo is very difficult; therefore there have been few studies on this subject. We found that the increased lymphocytes after ex vivo incubation for two to three weeks were significantly greater in recurrent ovarian carcinoma than in primary ovarian carcinoma, suggesting that immunity, and hence lymphocyte proliferation, is more strongly suppressed at initial states of tumor. Notably, the proportion of CD4+ T lymphocytes increased with expansion, while the proportion of CD8+ T lymphocytes decreased because cytotoxic CD8+ T lymphocytes may be activated when there is stimulation of tumor cells. The proportion of NK cells after ex vivo expansion was not different significantly.
This study subdivided regulatory T lymphocytes, separating cells that expressed CD25high+ from CD4+CD25+ regulatory T lymphocytes and restricting the definition of regulatory T lymphocytes to FoxP3-positive cells. A number of previous studies have shown that CD4+CD25+ regulatory T lymphocytes increased in the peripheral blood and tumor tissues of cancer patients.16,18,22-27 Recently however, CD4+CD25high+ T lymphocytes are considered as an important separate subpopulation because they would be expected to avoid host immune surveillance more rapidly than other CD4+CD25+ T lymphocytes. Therefore, our results may be more valuable than past studies because of the precise definition for regulatory T lymphocytes as CD4+CD25high+Foxp3+ T lymphocytes. However, because the percentage of CD25high+Foxp3+ cells in the CD4+ T lymphocyte population is very low, the most important determinant of CD25high+Foxp3+ cell number is the quantity of CD4+ T lymphocytes. Thus, for more accurate comparison, we limited our analysis to samples in which the proportion of CD4+ T lymphocytes after incubation increased by at least 46% (the average). Using this approach, we found that the overall percentage of CD4+CD25high+FoxP3+ T lymphocytes increased significantly, from 0.32±0.17% before expansion to 0.72±0.42% after expansion. When analyzed by cancer stage, we found that the increase in the proportion of CD4+CD25high+FoxP3+ regulatory T lymphocytes was significantly higher in recurrent ovarian carcinoma (247±228%) than in primary ovarian carcinoma (16±89%).
In summary, the present study suggest that expansion of T lymphocytes from TIL or tumor associated lymphocytes (TAL) using IL-2 for adoptive immunotherapy may increase the proportion of regulatory T lymphocytes, neither cytotoxic T lymphocytes nor NK cells, especially in cases of recurrent ovarian carcinoma. Furthermore, functional assays need to be performed to obtain information about different immunities according to the disease state and techniques for suppressing regulatory T lymphocytes need to be explored to develop successful immunotherapy for patients with ovarian carcinoma.
Figures and Tables
Fig. 1
The proportion of each immune cells before and after ex vivo expansion. CD4+ T lymphocytes were increased and CD8+ T lymphocytes were decreased after ex vivo expansion. The change in NK cells was not significant statistically. CD4+CD25high+Foxp3+ regulatory T lymphocytes (last pair of bars) increased significantly after ex vivo expansion.

Fig. 2
The proportional change of immune cells in primary and recurrent ovarian carcinoma after ex vivo expansion. CD4+ T lymphocytes, CD8+ T lymphocytes and NK cells did not differ significantly between primary and recurrent ovarian carcinoma. CD4+CD25high+Foxp3+ regulatory T lymphocytes was significantly greater in recurrent ovarian carcinoma than in primary ovarian carcinoma.
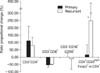
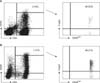
Fig. 3
Flow cytometry analysis of lymphocytes in primary and recurrent ovarian carcinoma after ex vivo expansion. (A) Representative primary ovarian carcinoma. (B) Representative recurrent ovarian carcinoma.
ACKNOWLEDGEMENTS
The authors are grateful to patients who participated in this study and several colleagues for collecting clinical materials and performing laboratory works.
Notes
References
1. Bremers AJ, Parmiani G. Immunology and immunotherapy of human cancer: present concepts and clinical developments. Crit Rev Oncol Hematol. 2000. 34:1–25.
2. Berek JS, Schultes BC, Nicodemus CF. Biologic and immunologic therapies for ovarian cancer. J Clin Oncol. 2003. 21:10 Suppl. 168s–174s.
3. O'Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004. 10:801–805.
4. Thompson C, Powrie F. Regulatory T cells. Curr Opin Pharmacol. 2004. 4:408–414.
5. Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004. 4:841–855.
6. Mittrucker HW, Kaufmann SH. Mini-review: regulatory T cells and infection: suppression revisited. Eur J Immunol. 2004. 34:306–312.
7. Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, et al. Immunologic tolerance maintained by CD25+CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001. 182:18–32.
8. Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003. 9:606–612.
9. Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J Exp Med. 2000. 192:295–302.
10. Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002. 168:1080–1086.
11. Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003. 4:337–342.
12. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003. 299:1057–1061.
13. Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004. 10:942–949.
14. Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002. 169:2756–2761.
15. Freedman RS, Tomasovic B, Templin S, Atkinson EN, Kudelka A, Edwards CL, et al. Large-scale expansion in interleukin-2 of tumor-infiltrating lymphocytes from patients with ovarian carcinoma for adoptive immunotherapy. J Immunol Methods. 1994. 167:145–160.
16. Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003. 98:1089–1099.
17. Elgert KD, Alleva DG, Mullins DW. Tumor-induced immune dysfunction: the macrophage connection. J Leukoc Biol. 1998. 64:275–290.
18. Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001. 193:1285–1294.
19. Ochsenbein AF, Sierro S, Odermatt B, Pericin M, Karrer U, Hermans J, et al. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001. 411:1058–1064.
20. Kono K, Salazar-Onfray F, Petersson M, Hansson J, Masucci G, Wasserman K, et al. Hydrogen peroxide secreted by tumor-derived macrophages down-modulates signal-transducing zeta molecules and inhibits tumor-specific T cell-and natural killer cell-mediated cytotoxicity. Eur J Immunol. 1996. 26:1308–1313.
21. Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970. 18:723–737.
22. Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002. 2:389–400.
23. Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25 high regulatory cells in human peripheral blood. J Immunol. 2001. 167:1245–1253.
24. Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005. 6:331–337.
25. Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999. 59:3128–3133.
26. Rosenberg SA. Immunotherapy and gene therapy of cancer. Cancer Res. 1991. 51:18 Suppl. 5074s–5079s.
27. Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, et al. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002. 168:4272–4276.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download