Abstract
Toremifene is an anti-estrogen which has been shown to be effective in the treatment of breast cancer, and is thought to be a less uterotrophic agent than tamoxifen. The risk assessment concerning endometrial cancer has been inconclusive because of its rare use up to the mid-1990s. We report a case of an adenosarcoma, which is a very rare type of uterine malignancy, after toremifene treatment for 5 years in a breast cancer patient. After 1 year of toremifene use, the patient had a benign Mullerian adenofibroma. After an additional 4 years of toremifene treatment, the endometrial polypoid lesion was transformed into a Mullerian adenosarcoma. Although toremifene is a promising anti-estrogenic agent in the treatment of breast cancer patients, clinicians should not neglect the possibility of a uterine malignancy.
Tamoxifen therapy is recognized as standard adjuvant chemoprophylactic therapy for the management of breast cancer worldwide. The anti-estrogenic effect of tamoxifen on the breast reduces local relapses, and increases overall survival and disease-free survival.1 However, the estrogenic activity of tamoxifen on the female genital tract has induced a wide spectrum of endometrial and myometrial pathologies, such as uterine malignancies.1,2 Among the tamoxifen-induced uterine malignancies, there are malignant mixed Mullerian tumors (MMMTs), including Mullerian adenosarcomas, that represent 8% of all uterine sarcomas.3-5
Toremifene is another anti-estrogen that has been shown to be effective in the adjuvant treatment of breast cancer. Due to the rare use of toremifene prior to the mid-1990s, risk assessment for this drug concerning endometrial cancer has been inconclusive.6-8
We managed a case of adenosarcoma associated with toremifene use in a breast cancer patient. To our knowledge, Mullerian adenosarcomas of the uterus in association with toremifene therapy have not been previously described. We report this case due to its rarity.
A 69-year-old woman was diagnosed with breast cancer in May 2001. The patient underwent left breast-conserving surgery and axillary lymphadenectomy, and was diagnosed with estrogen receptor-positive T1N0M0 invasive ductal carcinoma. Six weeks later, complementary radiotherapy was administered at a total dose of 5,400 cGy. Two weeks post-operatively, the patient began receiving 40 mg/day of toremifene (Farestone), which was continued for 5 years and 3 months.
In September 2002, she visited a gynecologist for a routine check-up. On transvaginal sonography, the endometrial thickness was 0.4 cm and heterogeneous fluid collection was noted. Thereafter, we followed the patient regularly. One year later, the endometrial cavity was dilated to 2×3.5 cm on sonography, and an endometrial curettage showed a Mullerian adenofibroma (Fig. 1A). The patient was then followed up by sonography regularly without definite interval change until September 2009 when a 4×3.8 cm heterogeneous mass was noted in the endometrial cavity (Fig. 1B). On magnetic resonance imaging (MRI), the expanded endometrial cavity was filled with a heterogeneous lesion characterized by a high signal intensity on a T1-weighted image. This was thought to be endometrial cancer (Fig. 2). A hysteroscopy revealed a 4×4 cm white, smooth-surfaced tumor filling the endometrial cavity. We performed a hysteroscopic mass excision. Based on the pathologic examination, Mullerian adenosarcoma was identified and the patient underwent a total abdominal hysterectomy with bilateral salpingo-oophorectomy and pelvic lymph node dissection. Gross examination did not show a residual lesion. However, microscopic examination revealed a residual tumor limited to the endometrium without myometrial invasion. Pelvic lymphadenectomy was negative for 17 nodes. The patient was finally diagnosed with a Mullerian adenosarcoma (FIGO Ia) and no further adjuvant treatment was administered. We have followed the patient once a month after the surgery, and there is no evidence of recurrence to date.
The endometrial polypoid mass removed in 2004 measured 2×0.5 cm. Histologically, the polypoid mass was composed of irregularly shaped glandular structures and loose edematous fibrous stroma (Fig. 3A). The surface and the glandular epithelium were both either cuboidal or columnar with occasional cilia or mucinous changes. The epithelial cells and stroma were positive for estrogen and progesterone receptors (Fig. 3A), and the stroma was partly positive for CD10 and smooth muscle actin (SMA). Mitoses were not seen and Ki-67 labeling was <5%. The polypoid mass was more similar to a Mullerian adenofibroma than an endometrial polyp. The specimen obtained from the hysteroscopic mass resection was composed of multiple polypoid fragments of endometrium. The polypoid fragments were composed of glandular and stromal components. The surface and the glandular epithelium were both cuboidal and focally serous with cilia. The stroma was highly cellular, especially around the glandular structures, forming a collar pattern, and beneath the surface epithelium there was an intraluminal polypoid growth. The stroma showed cellular atypism and frequent mitoses (17/10 HPF) (Fig. 3B). In addition, there were smaller polypoid lesions that had features similar to the previous. The stroma around the glands was positive for CD10 (Fig. 3C), whereas the other areas were positive for SMA (Fig. 3D). Ki-67 labeling was 20% to 30%.
Tamoxifen is a non-steroidal anti-estrogen that is useful as an adjuvant treatment for breast cancer in cases that have positive receptors as it inhibits cancer growth. However, tamoxifen has estrogenic effects on the endometrium and it appears to stimulate growth of endometrial cancer. Long-term treatment with tamoxifen produces endometrial proliferative changes, such as uterine polyps, endometrial hyperplasia, and stromal alterations. The prolonged use of tamoxifen has been associated with malignant changes.2 The possible association between tamoxifen and endometrial cancer was first recognized in 1985. By far, the most common uterine malignancy in tamoxifen-treated patients is endometrial adenocarcinoma.3,4 Additionally, MMMTs, including Mullerian adenosarcomas, have also been reported to be related to tamoxifen therapy.3
Uterine sarcomas is a heterogeneous rare group of neoplasm with a 3-7% incidence among uterine cancers and a 1-3% incidence among all gynecological cancers. This type of cancer is divided into the following pathologic types: carcinosarcomas (50%), leiomyosarcomas (30%), endometrial stromal sarcomas (10-15%), and adenosarcomas (5-10%).2
The first case report describing an association between uterine sarcoma and tamoxifen was published in 1988. Since then, 68 cases of uterine sarcomas related to tamoxifen treatment have been reported. There have been 14 cases (18.5%) of adenosarcomas, which is a rare variant of a MMMT.2
Tamoxifen is the standard adjuvant therapy for breast cancer. Currently, there is growing concern regarding significant side effects in women using tamoxifen for a long period of time. Although tamoxifen is still the anti-estrogen of choice in the treatment of breast cancer patients, many clinicians consider toremifene to be a valid and safe alternative to tamoxifen in endocrine-responsive breast cancer, for similar anti-tumor effects and the possibility of a lower incidence of treatment-related endometrial malignancies.6,8-10
Toremifene is a triphenylethlene derivative related to tamoxifen. Toremifene has a high affinity for the estrogen receptor in breast cancer, is active against the breast cancer, and has an antitumor activity. Stenbygaard et al.10 and Pagani et al.9 affirmed that no significant difference in response rate, duration, survival, and toxicity in the treatment of patients with breast cancer has been shown for toremifene and tamoxifen.
In preclinical studies, toremifene has been shown to cause less DNA-adduct formation and to have less estrogenic activity in the endometrium.11 Harvey et al.,7 Ha et al.,8 and Holli et al.6 concluded that compared with tamoxifen therapy, toremifene therapy has a low incidence of secondary endometrial cancer. In 2008, however, the International Breast Cancer Study Group reported a different result. According to their report, the incidence of second primary uterine tumors with toremifene and tamoxifen was similar.11 Conclusive data on the uterine carcinogenic effect of toremifene is not yet available due to the limited number of long-term users of toremifene in prospective clinical trials.
For the same reason, the duration for induction of uterine cancer after toremifene treatment is not yet conclusive. MB Marttunen et al. suggested that at least in the first 2-3 years, toremifene treatment does not increase the risk of endometrial cancer.12 According to Ha et al.,8 the majority of uterine cancers developed after more than 1 year of treatment, and the annual hazard rate for endometrial cancer was 1.0 per 1,000 patient treatment years.
Adenosarcoma is a very rare uterine malignancy detected in this patient on toremifene therapy who has no risk factors for uterine malignancy other than old age. Furthermore, this case was preceded by a benign mullerian adenofibroma.
In conclusion, toremifene is a promising anti-estrogenic agent in the treatment of breast cancer patients. However, long-term clinical data regarding induction of uterine malignancies are needed. Clinicians should not neglect the possibility of a uterine malignancy with toremifene therapy. Gynecologic follow-up of women using long-term toremifene therapy for carcinoma of the breast is essential, as clinicians do for tamoxifen therapy. There is no controversy that a comprehensive evaluation, including a resectoscopic endometrial biopsy, as in our case, should be done to establish a precise diagnosis for patients who are suspected of having a uterine complication related to toremifene use.
Figures and Tables
Fig. 1
Sonography. (A) The endometrial cavity was dilated to 2×3.5 cm. The lesion was diagnosed as Mullerian adenofibroma. (B) A 4×3.8 cm-sized heterogeneous mass in the endometrial cavity that was diagnosed as Mullerian adenosarcoma.
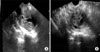
Fig. 2
The MRI finding for the same lesion as described in Fig. 1B. A heterogeneous lesion (arrow) thought to be endometrial cancer in the endometrial cavity with a high signal intensity on a T1-weighted image.
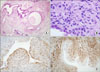
Fig. 3
Microscopic finding. (A) The first biopsy of an endometrial polypoid lesion was composed of irregularly shaped glandular structures and loose fibromatous stroma (H&E, ×40). (B) The second biopsy of the endometrial mass was composed of glandular or cystic structures with stromal proliferation with a collar pattern and an intraluminal polypoid growth pattern (H&E, ×40). (C) Immunohistochemical (IHC) staining for CD10 shows positive stromal cells around epithelial structures (IHC, ×100). (D) Immunohistochemical staining for smooth muscle actin shows a positive reaction in most of the stromal cells (IHC, ×100).
References
1. Konar H, Biswas PK, Choudhury P, Roy M. Gynecological follow up of women with long term tamoxifen therapy for carcinoma breast. J Obstet Gynecol India. 2006. 56:333–336.
2. Arenas M, Rovirosa A, Hernandez V, Ordi J, Jorcano S, Mellado B, et al. Uterine sarcomas in breast cancer patients treated with tamoxifen. Int J Gynecol Cancer. 2006. 16:861–865.
3. Arici DS, Aker H, Yildiz E, Tasyurt A. Mullerian adenosarcoma of the uterus associated with tamoxifen therapy. Arch Gynecol Obstet. 2000. 264:105–107.
4. Chourmouzi D, Boulogianni G, Zarampoukas T, Drevelengas A. Sonography and MRI of tamoxifen-associated mullerian adenosarcoma of the uterus. AJR Am J Roentgenol. 2003. 181:1673–1675.
5. Carvalho FM, Carvalho JP, Motta EV, Souen J. Mullerian adenosarcoma of the uterus with sarcomatous overgrowth following tamoxifen treatment for breast cancer. Rev Hosp Clin Fac Med Sao Paulo. 2000. 55:17–20.
6. Holli K, Valavaara R, Blanco G, Kataja V, Hietanen P, Flander M, et al. Safety and efficacy results of a randomized trial comparing adjuvant toremifene and tamoxifen in postmenopausal patients with node-positive breast cancer: Finnish Breast Cancer Group. J Clin Oncol. 2000. 18:3487–3494.
7. Harvey HA, Kimura M, Hajba A. Toremifene: an evaluation of its safety profile. Breast. 2006. 15:142–157.
8. Ha HK, Han W, Ko E, Kang SY, Lee JW, Cho J, et al. Toremifene as an adjuvant hormone therapy for estrogen receptor positive early breast cancer: therapeutic efficacy and effect on endometrium. J Breast Cancer. 2007. 10:258–262.
9. Pagani O, Gelber S, Price K, Zahrieh D, Gelber R, Simoncini E, et al. Toremifene and tamoxifen are equally effective for early-stage breast cancer: first results of International Breast Cancer Study Group Trials 12-93 and 14-93. Ann Oncol. 2004. 15:1749–1759.
10. Stenbygaard LE, Herrstedt J, Thomsen JF, Svendsen KR, Engelholm SA, Dombernowsky P. Toremifene and tamoxifen in advanced breast cancer: a double-blind cross-over trial. Breast Cancer Res Treat. 1993. 25:57–63.
11. Gianni L, Gelber S, Ravaioli A, Price KN, Panzini I, Fantini M, et al. Second non-breast primary cancer following adjuvant therapy for early breast cancer: a report from the International Breast Cancer Study Group. Eur J Cancer. 2009. 45:561–571.
12. Marttunen MB, Cacciatore B, Hietanen P, Pyrhonen S, Tiitinen A, Wahlstrom T, et al. Prospective study on gynaecological effects of two antioestrogens tamoxifen and toremifene in postmenopausal women. Br J Cancer. 2001. 84:897–902.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download