Abstract
Cervical cancer is the most common malignancy in Indian women. Cervical cancer usually spread by local extension and through the lymphatics to the retroperitoneal lymph nodes. Direct invasion of muscles by primary growth is more common than by metastatic involvement. We present a case of carcinoma of the cervix post radiotherapy to pelvis who on follow up presented with biceps muscle metastases as the initial sign of disseminated disease.
Metastasis of carcinoma cervix to the skeletal muscle is a rare event. Muscle is highly resistant to both primary and metastatic cancer. The cited factors for this high resistance include contractile activity, local changes in pH, oxygenation, the accumulation of lactic acid and other metabolites, blood flow per unit weight (mL/min/g), intramuscular blood pressure, and local temperature.1 Herewith presenting a rare event of biceps muscle metastasis as an initial sign of disseminated disease in cervix carcinoma. The patient was treated with local radiotherapy followed by palliative chemotherapy.
A 50 year lady, para-3, married since 30 years presented at our institute in June 2008 with a chief complaint of spasmodic pain in the lower abdomen with irregular menstrual cycles for 3 months. On general physical examination there were no inguinal or supraclavicular lymphadenopathy. On local examination the cervix was found to be replaced by ulcerative growth with obliteration of the right and left fornices, with induration felt along the upper vaginal wall. The left parametrium was thickened up to the pelvic wall. The right parametrium was involved medially. Biopsy from the cervix revealed moderately differentiated squamous cell carcinoma. Ultrasonography of the abdomen and pelvis revealed a 4.5×5 cm heterogeneous hypoechoic lesion in the region of cervix. Cystoscopy was negative for bladder infiltration. On proctoscopy, the rectal mucosa was normal. Chest X-ray was normal without evidence of metastasis. The patient was staged according to the FIGO classification system as stage IIIB carcinoma of the cervix. The patient was given radical radiotherapy course with whole pelvis external beam radiotherapy- 45 Gy/20# over 4 week time period followed by two sessions of intracavitary brachytherapy 900 cGy each (dose prescribed at point A), at weekly intervals. The patient received 4 cycles of weekly concurrent cisplatin (30 mg/m2 I/V) chemotherapy during the radiotherapy course. After concurrent chemoradiotherapy treatment the patient was free of disease and was on regular monthly follow up. In December 2008 she presented with a single painful swelling in the left arm, progressively increasing in size. On local examination a 4×3 cm size tender swelling was felt in the left biceps area, fixed to the underlying muscle, prominent on elbow flexion, and the overlying skin was free. On gynecological examination there was no evidence of local disease. On metastatic work up, contrast enhanced computed tomography (CECT) of the thorax revealed multiple small lung secondaries along with secondaries in the left biceps muscle (Fig. 1A, B). CECT abdomen and pelvis was normal. Fine needle aspiration cytology from the left arm swelling revealed a metastatic keratinizing squamous cell carcinoma (Fig. 1C, D). As a palliative measure for pain relief the patient was triaged for a short course of external radiotherapy 30 Gy in 10 fractions to the left arm. After two weeks of radiotherapy the patient responded well, the pain subsided and swelling also decreased in size (Fig. 2). The patient is on palliative chemotherapy with Cisplatin and Ifosfamide combination and doing well at the time of reporting.
Skeletal muscles represent 50% of the total body mass and receive a large percentage of total cardiac output. Despite this, haematogenous metastases to skeletal muscles have been reported to be rare. It is believed that muscle pH and muscle's contractile ability contribute to the resistance of skeletal muscles to metastatic disease.1 Cancer cell survival is found to be greater in denervated muscle that is unable to contract as opposed to electrically stimulated muscle.2
Malignancies known to frequently metastasize to the muscle are melanoma, kidney, lung and thyroid cancer; moreover skeletal muscle metastases have also been reported in lymphoma, leukemia and colon cancer patients. Psoas, iliopsoas, paraspinal muscles and proximal musculature of the upper and lower limbs represent the most frequently involved skeletal muscle sites.
Skeletal muscle involvement from cervical carcinoma is very rare, and usually documented in the context of an already far advanced stage tumor.3 To our knowledge till 2008, ten cases of skeletal muscle metastases have been reported in cervix carcinoma patients. The incidence of skeletal muscle metastases in cervix carcinoma is reported to be less than 1%. The most frequent site being the psoas muscle; however metastasis to masseter, intercostals, biceps and deltoid muscles have also been reported.4,5 The exact pattern of dissemination to the muscle is not clear from the available literature. Although various imaging studies have been used to identify metastasis to muscle, none are specific for differentiating among carcinomas, sarcomas or other muscle disorders. On unenhanced CT scans, muscle metastasis is revealed as an enlargement of the muscle. Occasionally, the findings may be subtle because the tumor is isodense to the surrounding muscle, and contralateral asymmetry is necessary to make the diagnosis. Also, radiopaque contrast administration is important to detect and evaluate the extent of the tumor. MR imaging findings of carcinoma metastatic to the muscle are not pathognomonic, and the differential diagnosis must include soft-tissue sarcoma, hematoma, and abscess. On MR imaging, metastatic lesions usually show poorly defined margins, low to intermediate signal intensity on TI-weighted images, high signal intensity on T2-weighted images, and enhancement with gadolinium.6
Differentiation between a primary soft tissue sarcoma and metastatic carcinoma is difficult without a biopsy. Metastases to skeletal muscles are frequently painful, whereas soft tissue sarcomas usually present as painless enlarging soft tissue masses.7
Although case reports are available, there is a lack of clear guidance for clinical management of such patients. The clinical outcome of patients with skeletal muscle metastasis is generally poor, given the common finding of diffuse metastatic disease. Treatment options may include radiotherapy, surgery or chemotherapy. Wide local excision if possible followed by local radiotherapy, or combined local radiotherapy (30 Gy/10#/300 cGy per fraction) and chemotherapy effectively controls 'pain' and the 'size' of the metastatic lesion in scenario of disseminated disease.8
In summary, as the incidence of skeletal muscle metastasis is less than 1% and due to the difficulty in differentiating from other benign lesions; it may be underreported in the literature. In a patient who has a history of cancer and presents with a painful soft-tissue mass, skeletal muscle metastasis must be included in the differential diagnosis, should be investigated properly with imaging and FNAC/biopsy. On confirmation of diagnosis, the patient should be offered treatment in the form of wide local excision, radiotherapy, or combined chemoradiotherapy. However, in the presence of skeletal muscle metastases in the setting of disseminated disease offers less hope for curative treatment.
Figures and Tables
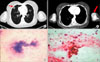 | Fig. 1(A) CECT thorax ill defined nodular opacities noted in bilateral lung parenchyma with the largest measuring 1.2×1 cm. (B) CECT left arm-ill defined 3.4×3.1 cm hypodense lesion in left biceps muscle. (C) Cluster of squamous cell carcinoma cells in a blood mixed fluid background, indicative of cystic change (H&E, ×10). (D) Cluster of keratinizing squamous cell carcinoma cells in a blood mixed fluid background (H&E, ×40). |
References
1. Acinas Garcia O, Fernandez FA, Satue EG, Buelta L, Val-Bernal JF. Metastasis of malignant neoplasms to skeletal muscle. Rev Esp Oncol. 1984. 31:57–67.
2. Weiss L. Biomechanical destruction of cancer cells in skeletal muscle: a rate-regulator for hematogenous metastasis. Clin Exp Metastasis. 1989. 7:483–491.
3. Ferrandina G, Salutari V, Testa A, Zannoni GF, Petrillo M, Scambia G. Recurrence in skeletal muscle from squamous cell carcinoma of the uterine cervix: a case report and review of the literature. BMC Cancer. 2006. 6:169.
4. Padhi S, Banerjee S, Das S, Rout N. Carcinoma cervix with atypical presentation of metastatic lesion as a cyst in the right deltoid muscle. Indian J Pathol Microbiol. 2008. 51:450–451.
5. Mariya Y, Watanabe S, Yokoyama Y, Tarusawa N, Takekawa S, Kattou K, et al. Metastasis of uterine cervical cancer to the biceps muscle of right upper arm; report of a case. Rinsho Hoshasen. 1990. 35:1447–1450.
6. Williams JB, Youngberg RA, Bui-Mansfield LT, Pitcher JD. MR imaging of skeletal muscle metastases. AJR Am J Roentgenol. 1997. 168:555–557.
7. Viswanathan N, Khanna A. Skeletal muscle metastasis from malignant melanoma. Br J Plast Surg. 2005. 58:855–858.
8. Tuoheti Y, Okada K, Osanai T, Nishida J, Ehara S, Hashimoto M, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004. 34:210–214.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download