Abstract
Objective
Mammalian target of rapamycin (mTOR) is known to promote cell proliferation, survival, and resistance to radiation. The aim of this study was to determine whether mTOR expression was associated with survival and the response to radiation in patients with cervical cancer.
Methods
After reviewing 119 patients treated by primary radiotherapy for stage IIB-IVA cervical cancer, a case-control study was performed. The cases (n=12) with local recurrence or radiation failure after primary radiation therapy were selected. For each case, two controls that had no recurrence were selected. Using pretreatment paraffin-embedded tissues, the cytoplasmic expression of phosphorylated-mTOR (p-mTOR) was evaluated by immunohistochemistry. Staining was scored based on intensity (intensity score [IS] 0-3) and proportion (proportion score [PS] 0-100). The progression free survival (PFS) was defined from the end of treatment to the day of recurrence by imaging studies or biopsy. The staining distribution and PFS were compared between the two groups. The results were analyzed by the Student t-test, Mann-Whitney U-test, Fisher's exact test, and Cox proportional hazards regression model.
Results
The p-mTOR cytoplasmic expression was significantly associated with a poor response to radiotherapy (p<0.01). With respect to survival, a higher cytoplasmic expression of p-mTOR was associated with a worse outcome (p=0.02). The hazard ratio for recurrence or radiation failure was 6.18 for mTOR IS and 1.04 for mTOR PS (p<0.05 for both), indicating that the degree of p-mTOR staining correlated with the recurrence risk.
Cervical cancer is the second most common cancer worldwide, resulting in approximately 275,000 deaths yearly.1 Although the incidence of cervical cancer in Korea has recently decreased, it ranks fifth among cancers in women, and 3,100 new patients were detected in 2004. Primary treatment for stage IIB-IVA cervical cancer is platinum-based concurrent chemoradiotherapy;2 this protocol has demonstrated a marked improvement in pelvic disease control. However, some patients experienced disease recurrence due to radioresistance. Identification of molecular biomarkers associated with the prognosis of patients with cervical cancer may help devise new treatment strategies to improve the clinical outcome of radioresistant cancer in patients.
Ogawa et al.3 reviewed the published reports describing microarray analysis to identify sets of genes that can be used for the characterization and prediction of response to radiotherapy in human cancers. These reports indicate that genes linked to cervical cancer are associated with DNA repair (XRCC5), apoptosis (BIK, SSI-3), signal transduction (MAP3K2: mitogen-activated protein kinase pathway), invasion and metastasis (CTSL, PLAU), hypoxia (HIF1A, CA12), and other functions (e.g., ALDH1).
A potential candidate biomarker related to signal transduction is the mammalian target of rapamycin (mTOR). It is also known as FKBP12 rapamycin-associated protein, rapamycin target, or sirolimus effector protein. mTOR is a serine/threonine-specific kinase responsible for mitogen-induced cell proliferation/survival signaling;4 it is activated by phosphorylation at Ser2448 by Akt via the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway and by autophosphorylation at Ser2481.5,6
mTOR has long been studied as a target molecule in several cancers. In biliary tract adenocarcinoma, patients with phosphorylated-mTOR (p-mTOR) positive tumors have a significantly shorter overall survival than those with p-mTOR negative tumors.7 In glioblastomas, high levels of expression of phosphorylated mitogen-activated protein kinase (p-MAPK) and activation of members of the Akt pathways are related to outcome, and the elevated expression of p-MAPK has been associated with increased radiation resistance, and represents an independent prognostic factor for this tumor.8
There are few published studies on mTOR expression and radioresistance in patients with cervical cancer. The observation that patients with advanced cervical cancer expressing Akt and mTOR, who are treated with cisplatin-based neoadjuvant chemotherapy, have a poor prognosis9 suggests that mTOR might be a potential new target for therapeutic intervention in patients with cervical cancer. Recently, the mTOR signaling pathway was reported to be activated in cervical carcinomas; inhibition of this pathway might be a potential therapeutic strategy for cervical cancers.10
In this study, p-mTOR expression in patients with stage IIB-IVA cervical cancer was evaluated using immunohistochemistry, and the correlation between the p-mTOR staining and survival was also investigated.
Between 1994 and 2004, 119 patients with stage IIB-IVA pathologically proven cervical cancer were treated by primary radiotherapy in the Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine. The medical records were reviewed according to institutional review board guidelines and a case-control study was performed. Thirty six patients were chosen according to completeness of their medical record and availability of a paraffin-block for evaluation.
The cases were selected from patients with local recurrence or radiation failure after primary radiation therapy (n=12). These cases were defined as the radio-resistant group. For each case, two controls were selected from patients who experienced no local recurrence for at least three years after radiation therapy (n=24). These patients were defined as the radio-sensitive group. Slides were prepared from stored pretreatment paraffin-embedded tissue blocks from these 36 patients.
All patients were treated with external beam radiotherapy (EBRT) and high-dose rate (HDR) intracavitary brachytherapy after consultation with a radiation oncologist. The whole pelvis total dose was 50.4 Gy (Gray) with 1.8 Gy daily fractions, administered five times a week. HDR brachytherapy was started 4 to 5 weeks after the initiation of EBRT. The dose of HDR brachytherapy was 24 Gy at point A, with 4 Gy per fraction twice a week for 3 weeks. After radiation treatment regular follow up was carried out. The progression free survival (PFS) was defined from the end of treatment to the day of recurrence by imaging studies or biopsy at the Samsung Medical Center.11
Immunohistochemical staining for p-mTOR was performed using the streptavidin-biotin method. The sections (4-µm thick) were deparaffinized with xylene, rehydrated, and incubated with fresh 0.3% hydrogen peroxide in methanol for 30 minutes at room temperature. The specimens were rehydrated through a graded ethanol series and washed in phosphate-buffered saline (PBS). After a blocking treatment, the specimens were incubated with anti-p-mTOR antibodies (Cell Signaling Technology, Beverly, MA, USA) at a dilution of 1:200 in PBS containing 1% bovine serum albumin (BSA) at 4℃ overnight. The sections were then washed with PBS and incubated in secondary antibody for 30 minutes at room temperature. The chromogen was a 3.3-0.02% solution containing 0.005% H2O2 in a 50 mM ammonium acetate-citric acid buffer, pH 6.0. The tissue sections were lightly counterstained with hematoxylin and then examined by light microscopy. There was no detectable staining in the negative controls prepared by omitting the primary antibody.9
Staining was evaluated by two independent observers without knowledge of the clinical outcome. One gynecologic pathologist (COS) and one gynecologic oncologist (TJK) reviewed the blinded slides and evaluated the immunohistochemical data independently. The staining intensity was scored as follows: 0, no appreciable staining in tumor cells; 1, barely detectable staining in the cytoplasm, nucleus, or membrane, compared with stromal elements; 2, readily detectable brown staining distinctly marking the tumor cell cytoplasm, nucleus, or membrane; and 3, dark brown staining in tumor cells completely obscuring the cytoplasm, nucleus, or membrane.12 The staining proportion was calculated according to the percent of the stained region. The staining was scored based on intensity (0-3) and proportion (0-100) (Fig. 1).
Statistical calculations were carried out using SAS ver. 9.1.3 (SAS Institute Inc., Cary, NC, USA). For the continuous variables, the student t-test was used for the variables that were normally distributed, and the Mann-Whitney U-test for the variables that were not normally distributed. Fisher's exact test was performed for the categorical variables. To evaluate the relationship of the p-mTOR staining to the PFS, the Cox proportional hazards regression model was used.
The patient characteristics are described in Table 1. The median follow-up for the patients studied was 49 months. At the time of analysis, all case patients were dead from causes related to the cervical cancer, and all control patients were alive with no recurrence after adjuvant treatment. There were no statistical differences in age, cell type, and FIGO stage between the two groups. The results of the immunohistochemical expression of p-mTOR in the 36 cervical squamous cell carcinomas (12 radio-resistant and 24 radio-sensitive) are summarized in Tables 2 and 3. There is only one case with weak staining in the radio-resistant group. However, there was no case of very strong staining (3+) in the radio-sensitive group. Therefore, expression of p-mTOR was significantly more frequent in the radio-resistant group compared to the radio-sensitive group (p<0.05), indicating that p-mTOR expression was associated with a poor response to radiation.
In Table 3, the PFS of all patients was analyzed based on p-mTOR expression. The median PFS was 9 months for patients in the radio-resistant group and 73 months for the patients in the radio-sensitive group. The hazard ratio of recurrence or radiation failure was 6.18 for mTOR intensity score (IS) and 1.04 for mTOR proportion score (PS) (p<0.05 for both) (Table 3), indicating that p-mTOR staining correlates with recurrence risk and might be a useful prognostic marker. With an increase in the intensity score by one, the case group was about at six times higher risk than the control group for recurrence of the cervical cancer. Fig. 1 shows the distribution of cells for p-mTOR staining; the expression of p-mTOR was detected in the cytoplasm. The patients with strong p-mTOR staining (IS=2,3) had a poorer prognosis (p<0.01) than the patients with weak p-mTOR staining (IS=0,1) (Fig. 2).
Histological evaluation of p-mTOR expression in patients with cervical cancer prior to treatment with primary radiotherapy showed a significant association between cytoplasmic p-mTOR expression and local recurrence or radiation failure. In addition, the correlation between the degree of staining and risk of recurrence suggests clinical significance and the potential for use as a prognostic marker.
The underlying mechanism of the association between p-mTOR expression and radioresistance is unknown. However, there are several clues from studies on mTOR and radiation treatment in patients with prostate and breast cancer.13,14 Bianco et al.15 showed that inhibition of mTOR causes anti-tumor activity in epidermal growth factor receptor (EGFR)-resistant cancer cell lines and xenografts; this effect appeared to be mediated by inhibition of survival signaling pathways and angiogenesis. The combination of everolimus (an mTOR inhibitor) with an EGFR inhibitor (cetuximab, gefitinib) potentiates this effect, and might re-sensitize resistant cancer cells to EGFR antagonists. In light of the recent approval of the mTOR inhibitors temsirolimus, everolimus, and AP23573, these results support the clinical application of anti-EGFR agents and mTOR inhibitors in combination. In breast cancer, a beneficial effect of combination treatment with the mTOR inhibitor RAD001 (everolimus) and letrozole has been reported.16 Moreover, mTOR specific siRNA showed the possibility of an alternative gene therapy approach in one study. Together with the findings of this study, these reports raise the possibility that a therapeutic agent used against the molecular target p-mTOR may be added to primary radiation in patients with cervical cancer for better control of disease recurrence. Although the sample size is small, these preliminary findings suggest the need for a larger study to confirm the results. In Table 3 and Fig. 2, the relationship between the clinical recurrence data and the mTOR staining of samples is illustrated. In addition, the results show the close relationship between mortality from cervical cancer and mTOR expression.
The limitations of this study include the following. The small sample size and retrospective study design make generalization to the general population difficult. However, the observations from this small series should prompt a large sample investigation including in vitro testing. Moreover, the association of p-mTOR expression with radioresistance may also reflect chemoresistance, which was not addressed in this study. This also made it difficult to perform multivariate analyses to evaluate p-mTOR expression as a predictor for radiation response. Since concurrent chemoradiation treatment is the standard treatment modality for advanced cervical cancer, the effects of radioresistance versus chemoresistance could not be therefore completely differentiated. However, since the majority of the study population (11 in the radio-resistant group, 19 in the radio-sensitive group) received radiation only, the study provides relatively solid evidence on the relationship between p-mTOR expression and radioresistance. In addition, other factors associated with the mTOR signal pathway were not studied.
This study investigated whether p-mTOR expression measured in pre-treatment biopsy specimens of human cervical cancers was associated with response to radiation. The result showed that pretreatment p-mTOR expression was significantly associated with local control of the cancer and PFS, raising the possibility that p-mTOR may be useful as a prognostic marker for patients with cervical cancer. Further studies with a larger sample size are needed to more clearly evaluate the predictive role of p-mTOR in the radiation response of patients with cervical cancer.
Figures and Tables
Fig. 1
Proportion score (PS) and intensity score (IS) of mammalian target of rapamycin (mTOR) expression. A, 0% (0); B, 5% (1); C, 80% (2); D, 85% (3).
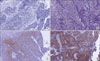
Fig. 2
Univariate progression-free survival (PFS) analysis of phosphorylated- mammalian target of rapamycin (p-mTOR) expression in 36 patients with locally advanced cervical cancer; mTOR strong staining group (intensity score [IS]=2,3) had a poorer prognosis for progression free survival (PFS) (p=0.02).

ACKNOWLEDGEMENTS
This work was supported by the Samsung Biomedical Research Institute grant, #SBRI C-A7-414-1.
References
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005. 55:74–108.
2. Petignat P, Roy M. Diagnosis and management of cervical cancer. BMJ. 2007. 335:765–768.
3. Ogawa K, Murayama S, Mori M. Predicting the tumor response to radiotherapy using microarray analysis (Review). Oncol Rep. 2007. 18:1243–1248.
4. Abraham RT, Gibbons JJ. The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res. 2007. 13:3109–3114.
5. Peterson RT, Beal PA, Comb MJ, Schreiber SL. FKBP12-rapamycin-associated protein (FRAP) autophosphorylates at serine 2481 under translationally repressive conditions. J Biol Chem. 2000. 275:7416–7423.
6. Nave BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999. 344 Pt 2:427–431.
7. Herberger B, Puhalla H, Lehnert M, Wrba F, Novak S, Brandstetter A, et al. Activated mammalian target of rapamycin is an adverse prognostic factor in patients with biliary tract adenocarcinoma. Clin Cancer Res. 2007. 13:4795–4799.
8. Pelloski CE, Lin E, Zhang L, Yung WK, Colman H, Liu JL, et al. Prognostic associations of activated mitogen-activated protein kinase and Akt pathways in glioblastoma. Clin Cancer Res. 2006. 12:3935–3941.
9. Faried LS, Faried A, Kanuma T, Sano T, Nakazato T, Tamura T, et al. Predictive and prognostic role of activated mammalian target of rapamycin in cervical cancer treated with cisplatin-based neoadjuvant chemotherapy. Oncol Rep. 2006. 16:57–63.
10. Ji J, Zheng PS. Activation of mTOR signaling pathway contributes to survival of cervical cancer cells. Gynecol Oncol. 2010. 117:103–108.
11. Lee JE, Huh SJ, Park W, Lim do H, Ahn YC, Park CS, et al. Radical radiotherapy for locally advanced cancer of uterine cervix. Cancer Res Treat. 2004. 36:222–227.
12. Freitas S, Moore DH, Michael H, Kelley MR. Studies of apurinic/apyrimidinic endonuclease/ref-1 expression in epithelial ovarian cancer: correlations with tumor progression and platinum resistance. Clin Cancer Res. 2003. 9:4689–4694.
13. Paglin S, Lee NY, Nakar C, Fitzgerald M, Plotkin J, Deuel B, et al. Rapamycin-sensitive pathway regulates mitochondrial membrane potential, autophagy, and survival in irradiated MCF-7 cells. Cancer Res. 2005. 65:11061–11070.
14. Cao C, Subhawong T, Albert JM, Kim KW, Geng L, Sekhar KR, et al. Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res. 2006. 66:10040–10047.
15. Bianco R, Garofalo S, Rosa R, Damiano V, Gelardi T, Daniele G, et al. Inhibition of mTOR pathway by everolimus cooperates with EGFR inhibitors in human tumours sensitive and resistant to anti-EGFR drugs. Br J Cancer. 2008. 98:923–930.
16. Awada A, Cardoso F, Fontaine C, Dirix L, De Greve J, Sotiriou C, et al. The oral mTOR inhibitor RAD001 (everolimus) in combination with letrozole in patients with advanced breast cancer: results of a phase I study with pharmacokinetics. Eur J Cancer. 2008. 44:84–91.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download