Abstract
Objective
This work investigates the role of glutathione S-transferase M1 (GSTM1), glutathione S-transferase T1 (GSTT1), and glutathione S-transferase P1 (GSTP1) enzymes and polymorphisms, which are found in phase II detoxification reactions in the development of cervical cancer.
Methods
This study was conducted with 46 patients diagnosed with cervical cancer and 52 people with no cancer history. Multiplex PCR methods were used to evaluate the GSTM1 and GSTT1 gene polymorphism. However, the GSTP1 (Ile105Val) gene polymorphism was studied using a PCR-RFLP method. The patient and control groups were compared using a chi-square test with p<0.05.
Results
In the patient group, statistical significance was determined for gravidity (p=0.03), parity (p=0.01), and the number of living children (p=0.01) compared to the control group. The gene frequency of GSTM1, GSTT1, and GSTP1 polymorphisms was evaluated. We observed that GSTM1 and GSTT1 null genotype frequencies were 54.3% and 32.6% respectively, while GSTP1 (Ile/Val), (Ile/Ile), (Val/Val) genotype frequencies were 52%, 44%, and 4%, respectively, in the cervical cancer patients. No statistical variation was determined between the control and patient groups in terms of GSTM1, GSTT1, and GSTP1 polymorphisms (p>0.05).
Cervical cancer is one of the most common types of cancer observed in women. The largest contributing factor in its development is the progression of untreated, high-risk types of human papillomavirus (HPV) in cervical tissue. Oncoproteins E6 and E7 have been shown to be activated by HPV, disrupting the normal cell cycle and DNA structures and leading to the development of cervical cancer.1-3 Epidemiological studies have shown that certain etiological factors other than HPV may also play a role in the development of cervical cancer. These may include marrying at a very early age, early childbirth, multiple births, low socio-economic status and heavy cigarette consumption.4-7 Smoking cigarettes may also play a role in the development of high-risk human papillomaviruses (HR-HPVs), which appear to interact with active cigarette smoking to increase the risk of high-grade cervical squamous intraepithelial lesions (HSIL).8,9 The nicotine in cigarettes has been found in cervical mucus, which may have a mutagenic impact.4,10,11
GST enzymes, which are encoded by GST genes, are responsible for the detoxification of chemicals found in the environment and naturally synthesized metabolites, and they play an important role in protecting tissue from oxidative damage. An increase or decrease in the tendency of certain types of cancer observed in a group of individuals is often linked to the genetic polymorphism observed in enzymes that play a role in the detoxification of xenobiotics. A significant relationship is observed between the risk of developing cancer and xenobiotic metabolism enzyme gene polymorphism. This relationship has highlighted the role of genetics in cancer etiology.12-14 The relation between GST gene polymorphism and cervical cancer has been investigated in various studies, which demonstrated that the risk of cervical cancer increases in women with GST gene polymorphism.15,16
In this study, we aimed to investigate the relationship between the development of cancer and general polymorphisms that play a role in detoxification reactions. Specifically, glutathione S-transferase M1 (GSTM1), glutathione S-transferase T1 (GSTT1), and glutathione S-transferase P1 (GSTP1) polymorphisms were studied in Turkish patients with cervical cancer.
This study was conducted as a prospective study in the Department of Obstetrics and Gynecology at the Medical School of Uludag University between 2008 and 2009. In this study, the patient group consisted of 46 cases diagnosed with cervical cancer, and the age-matched control group consisted of 52 cases with no cancer history. Both groups visited our clinic during same period. Members of both the patient group and the control group were asked to sign an informed consent form.
Blood samples from both the patient and the control groups were taken in EDTA tubes. DNA isolation was performed according to the procedures of the Dr. Zeydanlı (DZ) DNA isolation kit, and samples were stored at -20℃ until PCR. Multiplex PCR method was used to determine GSTM1 and GSTT1 polymorphisms in the isolated DNAs. For the GSTT1 polymorphism, forward 5'-TTCCTTACTGGTCCTCACATCTC-3' and reverse 5'-TCACCGGATCATGGCCAGCA-3' primers were used. For the GSTM1 polymorphism, forward 5'-GAACTCCCTGAAAAGCTAAAGC-3' and reverse 5'-GTTGGGCTCAAATATACGGTGG-3' primers were used. Albumin forward 5'-GCCCTCTGCTAACAAGTCCTAC-3' and reverse 5'-GCCCTAAAAAGAAAATCCCCAATC-3' primers were used as internal controls.17 Albumin 350 bp, GSTM1 219 bp and GSTT1 459 bp PCR products were formed. PCR conditions required denaturation for 5 minutes at 94℃ and then 35 cycles as follows: 1 minute at 94℃ (denaturation), 1 minute at 58℃ (annealing), one minute at 72℃ (elongation) and finally 10 minutes at 72℃ (final elongation). Genotypes were determined by migration of the products in agarose gel with added 2% ethidium bromide (Fig. 1).
GSTP1 (Ile105Val) gene polymorphism was determined using the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. For the GSTP1 polymorphism, forward 5'-ACCCCAGGGCTCTATGGGAA-3' and reverse 5'-TGAGGGCACAAGAAGCCCCT-3' primers were used.17 To identify the GSTP1 (Ile105Val) gene polymorphism among the products, the Alw 26 I (Genemark, Russia) enzyme was used. In the analysis conducted in 4% agarose gel after cutting the enzyme, genotypes were determined as follows: if the 176 bp PCR product from the GSTP1 gene was cut into two distinct products of 85 bp and 91 bp, then the genotype was identified as Val/Val; if three distinct products formed as 176 bp, 91 bp and 85 bp, then the genotype was identified as Ile/Val; and if the product was 176 bp then the genotype was identified as Ile/Ile (Fig. 2).
The characteristics of the study population are shown in Table 1. The age distribution was no different between patients and controls, the mean age being 53.73±10.35 and 51.32±8.86 years for patients and controls, respectively (p=0.11). No significant difference was seen between the two groups in terms of year of menopause and abortus. However, gravida, parity and number of living children were significantly different in the cervical cancer group and control group, respectively (p=0.03, p=0.01, and p=0.01).
Table 2 shows the distribution of GSTM1, GSTT1, and GSTP1 (Ile105Val) genotype prevalence in patients and controls. We found a prevalence of GSTM1 null genotype in 54.3% of patients compared with 57.7% in controls. The homozygous null genotype GSTT1 was found in 32.6% of patients and in 30.8% of controls. When the groups were compared in terms of GSTT1 and GSTM1 genotype, no statistical significance was determined (p=0.84 and p=0.73, respectively). Using subject with the Val/Val homozygote as a reference group, we found no association between the Ile/Ile and Ile/Val genotypes and the risk of cervical cancer after statistical analysis (p=0.730 and p=0.053, respectively). The prevalence of Ile allele was 71.8% and 70.1% in the cervical cancer group and control group, respectively.
Genetic variation in susceptibility to chemical carcinogens among individuals is one of the main factors leading to cancer development among human beings. Genetic variations among the genes forming the enzymes that play a role in metabolism, such as CYP and GST, have been shown to lead to susceptibility to the development of various cancers.18,19 The mu and theta classes of GST isozymes (GSTM1 and GSTT1, respectively) have a common and broad range of substrate specificities, and they detoxify the reactive metabolites of benzo-a-pyrene and other polycyclic aromatic hydrocarbons.12,20 The null genotype developing from homozygote deletion of GSTM1 or GSTT1 genes is frequently observed in lung and bladder cancers.21-26
When patients with cervical cancer were reviewed, 54.3% of them showed the GSTM1 null genotype. The GSTM1 null genotype among the control group was 57.7%. While the GSTM1 null genotype rate among the patients was lower than that of the control group, this difference is not statistically significant (p=0.73). On the other hand, while the GSTT1 null genotype rate among patients with cervical cancer was determined to be 32.6%, it was determined to be 30.8% in the control group. While the GSTT1 null genotype rate among patients was higher than that of the control group, this difference is not statistically significant (p=0.84).
Our study seems to be more in compliance with the studies of Warwick et al.,27 de Carvalho et al.,28 Settheetham-Ishida et al.,29 and Chen et al.,30 which did not demonstrate any relationship between the GSTM1 and GSTT1 null genotype and cervical cancer (p>0.05). Warwick et al.27 reported that no difference was found in the frequency of GSTM1 and GSTT1 null genotypes between controls and cervical carcinoma cases, including cervical intraepithelial neoplasia. Chen et al.30 did not observe any increase in incidences of cervical cancer in patients with the GSTM1 null genotype. In the study conducted by Sharma et al.31 on Indian patients with cervical cancer, the combined analysis of both GSTM1 null and GSTT1 null genotypes did not appear to influence the susceptibility. Contrary to these studies, Kim et al.12 reported that, among patients who carried HPV, the risk of developing cervical cancer before 40 is high (p<0.05) (Table 3).
The presence of the GSTP1 (Ile105Val) polymorphism produced no difference between the patient and control groups. Jee et al.32 reported that the polymorphism of GSTP1 in women who smoke cigarettes was associated with a higher risk of developing cervical cancer. In a study by Sobti et al.,4 it was reported that, in women with GSTM1 (null), GSTP1 (null) and GSTP1 (Ile105Val) genotype, the cervical cancer development rate was elevated in passive smokers. In our study, no relation could be found between the GSTP1 polymorphism and the development of cervical cancer. Since the number of cases in our study was limited, our findings would need to be supported with studies conducted on a larger number of cases (Table 4).
To conclude, demonstrating a relation between cancer types and genes that code the enzymes that act in detoxification metabolism is of great importance for determining risk groups for various cancer types. While the GST polymorphism is related to the development of cervical cancer in certain studies, no relation is seen in other studies. Many more studies should be conducted with larger patient and control groups to resolve this conflict.
Figures and Tables
Fig. 1
A representative multiplex PCR analysis of glutathione S-transferase (GST) polymorphisms. Albumin (350 bp), glutathione S-transferase T1 (GSTT1; 459 bp) and glutathione S-transferase M1 (GSTM1; 219 bp) genes PCR products resolved by agarose gel electrophoresis. Line MARKER is 100 bp DNA ladder. Lines 1 and 4 are both GSTT1 and GSTM1 present genotype, line 2 both GSTT1 and GSTM1 null genotype, line 3 is GSTT1 present genotype and GSTM1 null genotype.
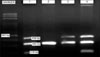
Fig. 2
Photograph of the PCR products of the glutathione S-transferase P1 (GSTP1) gene after Alw 26 I enzyme cutting and on 3.5% agarose gel. Line MARKER shows the 100 bp DNA ladder, line 1 and 4 shows individuals with Ile/Ile genotype (176 bp), line 2 and 5 shows Ile/Val genotype (176 bp, 91 bp, 85 bp), and line 3 shows the Val/Val genotype (91 bp, 85 bp).

Table 3
Frequency of glutathione S-transferase M1 (GSTM1) and glutathione S-transferase T1 (GSTT1) null genotypes in cervical cancer studies

*GSTM1 (null) (odds ratio [OR], 7.0; 95% confidence interval [CI], 2.19 to 22.36; p=0.0005) and GSTT1 (null) (OR, 10.2; 95% CI, 1.23 to84.18; p=0.02). †Null genotypes of GSTT1 and GSTM1 in cervical carcinoma were significantly overrepresented in the younger age subgroup (age 40 years or younger) compared with those of controls. ‡NS: not significant (p>0.05).
References
1. Pate Capps N, Stewart A, Burns C. The interplay between secondhand cigarette smoke, genetics, and cervical cancer: a review of the literature. Biol Res Nurs. 2009. 10:392–399.
2. Moodley M. Update on pathophysiologic mechanisms of human papillomavirus. Curr Opin Obstet Gynecol. 2005. 17:61–64.
3. Trunk MJ, Wentzensen N, von Knebel Doeberitz M. Molecular pathogenesis of cervical cancer and its first steps. Pathologe. 2005. 26:283–290.
4. Sobti RC, Kaur S, Kaur P, Singh J, Gupta I, Jain V, et al. Interaction of passive smoking with GST (GSTM1, GSTT1, and GSTP1) genotypes in the risk of cervical cancer in India. Cancer Genet Cytogenet. 2006. 166:117–123.
5. La Vecchia C, Negri E, Franceschi S, Parazzini F. Long-term impact of reproductive factors on cancer risk. Int J Cancer. 1993. 53:215–219.
6. Capalash N, Sobti RC. Epidemiology of cervical cancer: a case control study on north Indian population. Indian J Cancer. 1999. 36:179–185.
7. Winkelstein W Jr. Smoking and cancer of the uterine cervix: hypothesis. Am J Epidemiol. 1977. 106:257–259.
8. Settheetham-Ishida W, Singto Y, Yuenyao P, Tassaneeyakul W, Kanjanavirojkul N, Ishida T. Contribution of epigenetic risk factors but not p53 codon 72 polymorphism to the development of cervical cancer in Northeastern Thailand. Cancer Lett. 2004. 210:205–211.
9. Coker AL, Bond SM, Williams A, Gerasimova T, Pirisi L. Active and passive smoking, high-risk human papillomaviruses and cervical neoplasia. Cancer Detect Prev. 2002. 26:121–128.
10. Winkelstein W Jr. Smoking and cervical cancer: current status: a review. Am J Epidemiol. 1990. 131:945–957.
11. Gram IT, Austin H, Stalsberg H. Cigarette smoking and the incidence of cervical intraepithelial neoplasia, grade III, and cancer of the cervix uteri. Am J Epidemiol. 1992. 135:341–346.
12. Kim JW, Lee CG, Park YG, Kim KS, Kim IK, Sohn YW, et al. Combined analysis of germline polymorphisms of p53, GSTM1, GSTT1, CYP1A1, and CYP2E1: relation to the incidence rate of cervical carcinoma. Cancer. 2000. 88:2082–2091.
13. Malats N, Camus-Radon AM, Nyberg F, Ahrens W, Constantinescu V, Mukeria A, et al. Lung cancer risk in nonsmokers and GSTM1 and GSTT1 genetic polymorphism. Cancer Epidemiol Biomarkers Prev. 2000. 9:827–833.
14. Reszka E, Wasowicz W, Gromadzinska J. Genetic polymorphism of xenobiotic metabolising enzymes, diet and cancer susceptibility. Br J Nutr. 2006. 96:609–619.
15. Joseph T, Chacko P, Wesley R, Jayaprakash PG, James FV, Pillai MR. Germline genetic polymorphisms of CYP1A1, GSTM1 and GSTT1 genes in Indian cervical cancer: associations with tumor progression, age and human papillomavirus infection. Gynecol Oncol. 2006. 101:411–417.
16. Singh H, Sachan R, Devi S, Pandey SN, Mittal B. Association of GSTM1, GSTT1, and GSTM3 gene polymorphisms and susceptibility to cervical cancer in a North Indian population. Am J Obstet Gynecol. 2008. 198:303.e1–303.e6.
17. Abbas A, Delvinquiere K, Lechevrel M, Lebailly P, Gauduchon P, Launoy G, et al. GSTM1, GSTT1, GSTP1 and CYP1A1 genetic polymorphisms and susceptibility to esophageal cancer in a French population: different pattern of squamous cell carcinoma and adenocarcinoma. World J Gastroenterol. 2004. 10:3389–3393.
18. Idle JR. Is environmental carcinogenesis modulated by host polymorphism? Mutat Res. 1991. 247:259–266.
19. Nebert DW. Role of genetics and drug metabolism in human cancer risk. Mutat Res. 1991. 247:267–281.
20. Mannervik B, Danielson UH. Glutathione transferases: structure and catalytic activity. CRC Crit Rev Biochem. 1988. 23:283–337.
21. Nakachi K, Imai K, Hayashi S, Kawajiri K. Polymorphisms of the CYP1A1 and glutathione S-transferase genes associated with susceptibility to lung cancer in relation to cigarette dose in a Japanese population. Cancer Res. 1993. 53:2994–2999.
22. Zhong S, Howie AF, Ketterer B, Taylor J, Hayes JD, Beckett GJ, et al. Glutathione S-transferase mu locus: use of genotyping and phenotyping assays to assess association with lung cancer susceptibility. Carcinogenesis. 1991. 12:1533–1537.
23. Brockmoller J, Kerb R, Drakoulis N, Staffeldt B, Roots I. Glutathione S-transferase M1 and its variants A and B as host factors of bladder cancer susceptibility: a case-control study. Cancer Res. 1994. 54:4103–4111.
24. Hosgood HD 3rd, Berndt SI, Lan Q. GST genotypes and lung cancer susceptibility in Asian populations with indoor air pollution exposures: a meta-analysis. Mutat Res. 2007. 636:134–143.
25. Shi X, Zhou S, Wang Z, Zhou Z. CYP1A1 and GSTM1 polymorphisms and lung cancer risk in Chinese populations: a meta-analysis. Lung Cancer. 2008. 59:155–163.
26. Srivastava DS, Mishra DK, Mandhani A, Mittal B, Kumar A, Mittal RD. Association of genetic polymorphism of glutathione S-transferase M1, T1, P1 and susceptibility to bladder cancer. Eur Urol. 2005. 48:339–344.
27. Warwick A, Sarhanis P, Redman C, Pemble S, Taylor JB, Ketterer B, et al. Theta class glutathione S-transferase GSTT1 genotypes and susceptibility to cervical neoplasia: interactions with GSTM1, CYP2D6 and smoking. Carcinogenesis. 1994. 15:2841–2845.
28. de Carvalho CR, da Silva ID, Pereira JS, de Souza NC, Focchi GR, Ribalta JC. Polymorphisms of p53, GSTM1 and GSTT1, and HPV in uterine cervix adenocarcinoma. Eur J Gynaecol Oncol. 2008. 29:590–593.
29. Settheetham-Ishida W, Yuenyao P, Kularbkaew C, Settheetham D, Ishida T. Glutathione S-transferase (GSTM1 and GSTT1) polymorphisms in cervical cancer in Northeastern Thailand. Asian Pac J Cancer Prev. 2009. 10:365–368.
30. Chen C, Madeleine MM, Weiss NS, Daling JR. Glutathione S-transferase M1 genotypes and the risk of squamous carcinoma of the cervix: a population-based case-control study. Am J Epidemiol. 1999. 150:568–572.
31. Sharma A, Sharma JK, Murthy NS, Mitra AB. Polymorphisms at GSTM1 and GSTT1 gene loci and susceptibility to cervical cancer in Indian population. Neoplasma. 2004. 51:12–16.
32. Jee SH, Lee JE, Kim S, Kim JH, Um SJ, Lee SJ, et al. GSTP1 polymorphism, cigarette smoking and cervical cancer risk in Korean women. Yonsei Med J. 2002. 43:712–716.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download