Abstract
A 26-year-old girl was referred to us in December 2008 with progressive pelvic mass while on chemotherapy. In May 2008, she presented with large adnexal mass and high alpha-fetoprotein (AFP, 265.7 ng/mL; normal range, 0 to 10). She underwent laparoscopic right salpingo-oophorectomy with staging. Since histology was immature teratoma grade I, FIGO stage 1 she was kept on surveillance. In September 2008, she developed recurrent pelvic mass with AFP levels of 2,400 ng/mL. Three courses of chemotherapy (bleomycin-etoposide-cisplatin) were given. Post-chemotherapy AFP normalized but tumor size increased. CT-scan (abdomen-pelvis) showed a large pelvic mass with calcification specks; infiltrating the sigmoid colon and abdominal wall. With provisional diagnosis of growing teratoma syndrome she had exploratory laparotomy with excision of pelvic mass along with sigmoid colon, excision of right pelvic and subcutaneous deposits, omentectomy and sigmoid anastomosis. Left ovary, left tube and uterus appeared normal and were preserved. Histology of all masses showed mature teratoma, no immature elements. At six months follow up she is disease free and has resumed menstruation. Growing teratoma syndrome is a clinico-pathological presentation during/post-chemotherapy in malignant ovarian germ cell tumor where mature teratoma grows and requires complete surgical excision. Our case highlights the safety and adequacy concerns of laparoscopic management of malignant ovarian tumor. Literature review suggests good prospects of resumption of menses, child bearing and five year survival in case of growing teratoma syndrome.
Recurrent ovarian germ cell tumors in young women present a challenge for management since fertility preservation is the aim of therapy without compromising cures. Stage and histology of these tumors at primary presentation may predict the biology however the early relapses can occur in some. Further treatment of these relapses is chemotherapy which leads to complete regression in majority however in few it may progress to a mature teratoma.
DiSaia et al.1 in 1976 were the first to report this phenomenon as "chemotherapeutic retroconversion." Six years later Logothetis et al.2 observed similar clinical progression in males with germ cell tumor of testis and coined the term "growing teratoma syndrome." According to him by definition growing teratoma syndrome must exhibit- normalization of previously elevated tumor markers (alpha-fetoprotein [AFP] or beta human chorionic gonadotropin [HCG]), enlargement of tumor or finding a new tumor mass, and only mature teratoma element in pathologic examination. The radiological features include increased density of mass with well circumscribed margins, onset of internal calcification with fatty areas and cystic change.3
Meticulous search of English literature on PubMed (from 1975 to 2008) revealed only 40 cases and this is the first reported case of growing teratoma syndrome post laparoscopic management of germ cell tumor of ovary. All of them developed following surgery plus chemotherapy for malignant ovarian germ cell tumor. Commonest primary histology was immature teratoma (31/40 cases).
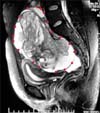
A 26-year-old unmarried lady was referred to us in December 2008 with progressive pelvic mass while on chemotherapy. In May 2008, during investigations for lump and pain in abdomen a large complex right ovarian mass was detected on MRI (Fig. 1). Serum tumor marker study showed CA125-235.8 U/mL (normal range, 0 to 35 U/mL); AFP, 265.7 ng/mL (normal range, 0 to 10 ng/mL), carcino-embryonic antigen (CEA) and beta HCG were normal. She underwent laparoscopic right salpingo-oophorectomy, omental biopsy with peritoneal lavage. Specimen was retrieved by increasing supra pubic port. Records neither mention about spillage during removal of mass nor the removal technique. No gross disease was left at the end of the surgery. Histology showed immature teratoma FIGO stage I G1 with presence of mature teratoma elements (from all three germ cells) and normal omental tissue. Peritoneal fluid cytology was negative. She was put on surveillance. There were no imaging studies done in immediate postoperative period.
Four months later in September 2008 she developed recurrent pelvic mass. Positron emission tomography and computer tomography (PET/CT) scan showed 2-[18 F] fluoro-2-deoxy-D-glucose (FDG) avid mass lesions in bilateral adenexa with multiple peritoneal and anterior abdominal wall deposits (Fig. 2A). Now her AFP level was 2,375.98 ng/mL. She received three courses of chemotherapy in form of bleomycin, etoposide and cisplatin. Following three cycles of chemotherapy AFP normalized but tumor size increased on clinical examination (Fig. 2B). CT scan (abdomen-pelvis) showed significantly increased size and calcification in pelvic mass, as well as increase in size of abdominal wall deposit (Fig. 2C). Later in December 2008 she was referred to us. With a working diagnosis of growing teratoma syndrome, we offered her surgical salvage with fertility preservation.
After thorough counseling she consented for exploratory laparotomy and excision of the mass with fertility preservation. She showed extreme keenness in fertility preservation. On laparotomy, mass was involving a portion of sigmoid colon however uterus, left ovary and tube were normal. Hence excision of mass with 17 cm sigmoid colon, right pelvic nodule, anterior abdominal mass, omentectomy, sampling from adhesions (Fig. 2D-specimen) and hand sewn sigmoid anastomosis was done. No macroscopic deposit was left at the end of surgery (RO state). Histology report revealed mature cystic teratoma, infiltrating the wall of sigmoid colon. Two of three pericolic nodes showed mature glial tissue deposits. The omentum, right pelvic nodule and uterine surface nodules showed deposits of mature teratoma. The subcutaneous nodule revealed mature glial tissue deposits. No immature elements or viable carcinoma were seen in any of the sections studied.
She has been on follow up with imaging and tumor markers, every three months. She resumed menstruation five months post op and is disease free at six months follow-up.
The possible pathogenesis of development of growing teratoma syndrome is either malignant cell differentiation into mature teratoma or selective chemotherapy induced destruction of immature elements.4 Further the chemotherapy may alter the cellular kinetics of totipotent malignant cells in some way to favour the development of mature elements rather than immature elements.5
Complete surgical excision of the mass is required to avoid pressure effects and potential malignant transformation to either sarcoma or carcinoma. Vascular thrombosis, ureteral obstruction, bowel obstruction, bile duct obstruction and fecal fistula have been reported as a result of pressure effects.4,6,7 Malignant transformation to sarcoma, adenocarcinoma or primitive neuroectodermal tumor is reported in 3% cases.6,8 Non-resectable masses could be treated with long term alpha-2 interferon but the regression is slow, incomplete and discontinuation results in progression of disease.4,9 The prognosis after complete surgical excision in both females and males is excellent. Only few deaths are reported in literature. (Nimkin et al.10 and Hariprasad et al.7). Andre et al.4 in his series of 30 cases of growing teratoma syndrome in males concluded that factors predicting development of this syndrome were presence of mature teratoma elements in primary tumor, incomplete resection of primary tumor and no reduction in tumor size after chemotherapy.
In our case mature elements from all three germ cell lines were present in primary tumor. Further, possibility of relapse due to spillage can not be ruled out (though record of capsule rupture and spillage not available). Moreover port site recurrence highlights the concern of laparoscopic management of complex ovarian masses in terms of safety and adequacy.
She resumed menstruation five months later and at six months follow up she is disease free, which indicates adequacy of the therapy. However it needs to be seen how she fares in terms of fertility and tumor recurrence. Further, hysterectomy and left salpingo-oophorectomy after completion of her family is desirable.
Figures and Tables
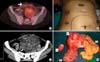 | Fig. 2(A) PET-CT pelvis showing fluoro-2-deoxy-D-glucose (FDG) avid pelvic mass and abdominal wall deposit (marked by white arrow). (B) Photograph showing abdomino-pelvic mass marked in blue. Black arrow shows abdominal wall mass (port site of specimen extraction), other ports marked by black ellipse. (C) CT scan abdomen pelvis post chemotherapy showing increased calcification in pelvic mass. (D) Specimen showing pelvic mass (M), two ends of sigmoid colon (S), anterior abdominal wall deposit (A), and omentum (O). |
References
1. DiSaia PJ, Saltz A, Kagan AR, Morrow CP. Chemotherapeutic retroconversion of immature teratoma of the ovary. Obstet Gynecol. 1977. 49:346–350.
2. Logothetis CJ, Samuels ML, Trindade A, Johnson DE. The growing teratoma syndrome. Cancer. 1982. 50:1629–1635.
3. Moskovic E, Jobling T, Fisher C, Wiltshaw E, Parsons C. Retroconversion of immature teratoma of the ovary: CT appearances. Clin Radiol. 1991. 43:402–408.
4. Andre F, Fizazi K, Culine S, Droz J, Taupin P, Lhomme C, et al. The growing teratoma syndrome: results of therapy and long term follow-up of 33 patients. Eur J Cancer. 2000. 36:1389–1394.
5. Dixon FJ, Moore RA. Armed Forces Institute of Pathology. Tumors of the male sex organs. Atlas of tumor pathology. 1952. Vol 8. Washington, DC: Armed Forces Institute of Pathology;316–332.
6. Williams SD, Blessing JA, DiSaia PJ, Major FJ, Ball HG 3rd, Liao SY. Second-look laparotomy in ovarian germ cell tumors: the Gynecologic Oncology Group experience. Gynecol Oncol. 1994. 52:287–291.
7. Hariprasad R, Kumar L, Janga D, Kumar S, Vijayaraghavan M. Growing teratoma syndrome of ovary. Int J Clin Oncol. 2008. 13:83–87.
8. Jumean HG, Komorowski R, Mahvi D, Anderson T. Immature teratoma of the ovary: an unusual case. Gynecol Oncol. 1992. 46:111–114.
9. Kattan J, Droz JP, Culine S, Duvillard P, Thiellet A, Peillon C. The growing teratoma syndrome: a woman with nonseminomatous germ cell tumor of ovary. Gynecol Oncol. 1993. 49:395–399.
10. Nimkin K, Gupta P, McCauley R, Gilchrist BF, Lessin MS. The growing teratoma syndrome. Paediatr Radiol. 2004. 34:259–262.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download