Abstract
Objective
Transrectal ultrasound (TRUS) has been widely used for guiding prostate implants, but not much for interstitial brachytherapy (IBT) of cervix cancer. The aim of our study is to report our experience with TRUS guided high dose rate (HDR) IBT in patients with carcinoma of uterine cervix.
Methods
During the year 2005-2006, 25 patients of cervical cancer not suitable for intracavitary radiotherapy (ICRT), were enrolled in this prospective study. We used B-K Medical USG machine (Falcon 2101) equipped with a TRUS probe (8658) having a transducer of 7.5 MHz for IBT. Post procedure, a CT scan was done for verification of needle position and treatment planning. Two weekly sessions of HDR IBT of 8-10 Gy each were given after pelvic external beam radiation therapy.
Results
A total of 40 IBT procedures were performed in 25 patients. Average duration of implant procedure was 50 minutes. There was no uterine perforation in any of 11 patients in whom central tandem was used. CT scan did not show needle perforation of bladder/rectum in any of the patients. During perioperative period, only 1 procedure (2.5%) was associated with hematuria which stopped within 6 hours. Severe late toxicity was observed in 3 (12%) patients. Overall pelvic control rate was 64%.
Carcinoma of uterine cervix is mainly treated by radiotherapy which consists of external beam radiation therapy (EBRT) and intracavitary radiotherapy (ICRT).1 Though ICRT is an integral part of treatment, certain patients are not suitable for it due to extensive disease in the cervix, obliteration of the cervical os, narrow vagina, extension of disease into the lower vagina and parametrical disease beyond the high dose range of the ICRT applicators. Interstitial brachytherapy (IBT) is used as an alternative to ICRT in such patients.2,3 IBT consists of placement of multiple needles in the pelvis through the perineum. Various types of transperineal templates are used to guide the implantation of needles and securing them.4,5 Even though IBT is a better option in such patients, the associated morbidity due to physical injury and radiation dose to organs at risk (OAR) still remains a concern. Various series on IBT in gynecological malignancies, primary as well as recurrent, have reported 4-21% risk of severe late toxicity like recto-vaginal fistulas.2 Recent studies6-8 with CT scan based planning systems and high dose rate (HDR) technology, the radiation related morbidity has reduced but there is significant risk of needle trauma to bladder, rectum and other pelvic structures during the procedure. Besides this, accurate placement of IBT needles in the target area is of utmost importance for good dosimetry. Use of imaging device during the procedure may assist in accurate placement of the needles for better target coverage and reduced physical injury as well as radiation injury to OAR. Various authors have used X-ray fluoroscopy,9 ultrasonography (USG),10 CT scan,6 MRI,11,12 laparoscopy,12,13 and laparotomy14 for guiding brachytherapy procedures in gynecological malignancies. Methods like CT/MRI scans have practical limitations and are rarely used. Transrectal ultrasound (TRUS) has been used for guiding the ICRT15 and prostate seed16 and IBT17 implants. It can provide the real time image of pelvic structures and the movement of implant needles and thus potentially help in avoiding the injury to OAR. It has many advantages in terms of easy availability, simple and cost effective equipment, free from ionizing radiations. The aim of our study is to report our experience with TRUS guided HDR IBT in patients with carcinoma of uterine cervix.
During the year 2005 and 2006, 25 patients with cervical cancer were enrolled in this prospective study. The various indications of IBT were as follows: 1) Patients who completed pelvic EBRT and not found suitable for ICRT due to large growth, narrow vagina and obliterated vaginal os (Group I); 2) Patients having persistent parametrial disease after completion of standard EBRT and ICRT treatment (Group II); and 3) Patients with recurrent cervical cancer who had previously received standard course of RT (Group III). Pretreatment workup included detailed physical examination, biopsy, and various radiological and endoscopic investigations as per the International Federation of Gynecology and Obstetrics (FIGO) staging system. The treatment schedules for different groups were as follows. Group I: whole pelvis EBRT with a dose of 50 Gy with conventional fractionation (last 10 Gy with split field) followed by two sessions of weekly IBT implant with a dose of 10 Gy each. Group II: Whole pelvis EBRT as in Group I followed by 3 sessions of weekly ICRT with 7 Gy to point A each session. This was followed by single session of IBT with 6 to 8 Gy depending upon the amount of residual disease. Group III: Whole pelvis EBRT dose of 30 to 45 Gy depending upon the interval between previous treatment and the recurrence. This was followed by 1 to 2 sessions of weekly IBT with a dose of 8 to 10 Gy each. Weekly chemotherapy with cisplatin 40 mg/m2 was given during EBRT to suitable patients.
The implant was performed using Martinez Universal Perineal Interstitial Template (MUPIT) under the guidance of TRUS imaging (Falcon 2101, BK Medicals, Herlev, Denmark). After administering spinal anesthesia, patient was put in the lithotomy position. Foley's tri-lumen urinary catheter was inserted. To start with, a thorough per vaginal and per rectum examination was done to assess the disease extent. After inserting a condom over the TRUS probe, it was introduced in the rectum and the bladder was identified first by visualizing the Foley's bulb so that orientation of other pelvic structures could be made. With the help of two buttons on the probe, both longitudinal and transverse images were visualized to assess the cranio-caudal and lateral extent of disease, if possible. Then, uterine sound was negotiated to check the patency of os and if desired, central tandem was introduced in the uterine cavity (Fig. 1). The obturator of the template assembly was slided over it in the vagina till it fitted against the cervix. If the uterine tandem was not to be used, then the obturator was inserted in the vagina and inner plate of the template was fitted over it and fixed with a screw. The plate was positioned against the perineal wall and stitched to the skin. The stainless steel needles (having blind ends) with 20 cm length were inserted one by one through the perineum from anterior to posterior aspect since the needles closer to TRUS probe could obscure the anterior needle images. The needle movement was tracked up to a desired depth by visualizing the tip of needle (Fig. 2) seen by moving TRUS probe in forward direction. Any needle causing accidental entry to bladder, rectum or bowel was immediately withdrawn (Fig. 2). Laterally, the needles were implanted till the lateral pelvic wall on either sides or the lateral extent of the target area based on the clinical and radiological findings. After inserting the desired numbers of needles for adequate coverage of the target area (Fig. 3), TRUS probe was taken out from the rectum and digital rectal examination was done to rule out any needle penetration in rectum. The needles were fixed with help of screws to prevent their movement. Outer plate of the template was fitted aligning the perineal plate and the two plates were fixed to each other with help of screws.
A planning CT scan of the whole pelvis was done with slice thickness of 2.5 mm. Brachytherapy planning was performed on PLATO planning system vers. 14.1 (Nucletron, Veenandaal, Netherlands) where target and OAR were delineated. The contour drawn by the line joining the outermost needles on the each CT slice constituted the boundary of the target (Fig. 4). However the cranio-caudal extent of the target was decided by selecting the length of needles keeping in mind the clinical and radiological findings. After finalizing the target and OAR volumes, implant needles were also marked on each slice in order to reconstruct the needle length. Using step size of 2.5 mm, a plan was generated (Fig. 4). The dose was not prescribed to a particular point; it was rather prescribed at the periphery of the target volume. Only dwell positions within the target volume were activated. If needed, both graphic and geometric optimization was done to achieve the best plan. After the plan approval, patient was taken to brachytherapy suite (Microselectron HDR remote after loading unit) for treatment. Implant needles were removed immediately after completion of the treatment. Routine antibiotics were prescribed for a period of 5 days.
Patients were followed every month till 6 months, then every 2 months till one year and then subsequently every 3 months till 2 years. At every visit, clinical examination was performed and, if necessary, CT/MRI scans, to assess the disease status and toxicity. PET scan was also done if there was a suspicion of disease on clinical/radiological examination. For assessment of late toxicity, Radiation Therapy Oncology Group criteria were used.
A total of 40 IBT procedures were performed in 25 patients. Various clinical characteristics of the patients are given in Table 1. Age ranged from 32 to 67 years (median, 48 years). Group I had 10 patients, Group II 6 and Group III had 9 patients. Table 2 shows various details of the implant procedure. Average duration of procedure (from insertion of TRUS probe to fixation of the both plates of template) was 50 minutes with a range of 40 to 75 minutes. Procedures with central tandem took relatively longer time since it required dilatation of cervical os. Bladder was very well visualized in every patient and hematuria was encountered in none of the procedures. An average of 18 needles was implanted in each patient. Average length of needle insertion beyond cervix/vault was 4 cm. In operated patients, this length was kept below 4 cm beyond the vault in order to avoid physical injury, during procedure, and late radiation injury to bowel. CT scan, which was done for planning, revealed no needle in the bladder or rectum in any of the patients. There was no uterine perforation in any of 11 patients whom central tandem was used. There was no hematuria during the implant procedure in the operative room. During periopertive period (within 24 hours of removal of the implant), only one patient had hematuria which was managed by continuous bladder irrigation and simple conservative methods. The hematuria stopped within 6 hours. Thus, only 1 out of 40 procedures (2.5%) was associated with hematuria.
Table 3 shows the various dosimetric indices for the target and OAR using prescription dose of 10 Gy. In general, rectum received higher doses than bladder. Severe late toxicity was observed in 3 (12%) patients. One patient had vesico-vaginal fistula and required diversion colostomy. The other 2 patients, one with bowel obstruction and another with Grade 3 proctitis, were managed conservatively.
Follow-up ranged from 3 to 20 months (median, 13 months). Overall pelvic control rate was 64%; and Group 1, Group II, and Group III had pelvic control rate of 80%, 50%, and 56%, respectively. Of 9 pelvic failures, 4 were central, 2 parametrial, 3 nodal. Three patients had associated distant metastases (1 each in para-aortic nodes, bones and supraclavicular fossa).
TRUS is widely used for prostate IBT but there is only one study in the literature so far pertaining to its use in IBT for cervical cancer.18 Our present study is the second study, after Stock et al.,18 in the literature. ICRT procedures are gradually becoming image based and there are recommendations19,20 for image guided ICRT. HDR IBT is still performed largely without any image assistance and there are no guidelines regarding the use of imaging for implant procedure, target volume delineation or planning. Compared to ICRT, IBT has higher risk of physical trauma during procedure and late complications. Therefore, use of a suitable imaging device during IBT is truly justified. Results of our study have shown that TRUS helps in avoiding the injury to OAR with good accuracy and precision during the IBT procedure.
In 1978, Brascho et al.21 first described the use of USG in planning ICRT. Subsequent studies22-24 reported the use of real time intraoperative USG mainly for correct placement of central tandem in the uterus and for preventing and detecting the uterine perforations during ICRT application. Fleischer et al.15 in 1990 first described the use of TRUS for intrauterine procedures. For IBT, TRUS has been tried in by Stock et al.18 so far. They reported, for the first time in 1997, their experience with TRUS guided IBT in 7 patients with advanced and recurrent gynecological malignancies. A total of 12 procedures were performed in these 7 patients. They used Bruel & Kjaer (B & K, Marlborough, MA, USA) ultrasound base unit (model 3535) and B & K biplanar ultrasound probe (model 8551 or 8558). Of these 7 patients, only 4 had cervical cancer, all stage IIIB. Their median procedure time was longer as compared to our study (130 minutes vs. 50 minutes). This could be due to larger number of patients in our study, allowing us to perfect this technique. In first few procedures in our study, time taken was as large as 75 minutes. Contrary to the common belief, use of TRUS does not prolong the procedure time. In our experience, as compared to manual method, it takes less time since TRUS helps in visualizing and identifying the pelvic structures and needles easily and accurately. Usually, longer time is needed for the procedure with the use of tandem and larger number of IBT needles. Even though, the median number of IBT needles in Stock et al.18 study and our study is same (18 needles), our procedure time was comparatively much shorter. Stock et al.18 did not report the accuracy of needle implantation though they have also performed CT scan based planning for some patients. In our study, all patients underwent CT scan for planning and none of the 40 procedures had shown presence of needle (s) in OAR, thereby proving the high accuracy of TRUS in avoiding injury to OAR. Though the study by Erickson et al.6 and Beriwal et al.8 used CT scan based planning for IBT, but did not use TRUS guidance for implant procedure. Erickson et al.6 reported CT scan confirmed needle perforation of pelvic structures in 8 out of 25 patients (32%) while Beriwal et al.,8 who used transabdominal ultrasound (TAUS) for implant procedure, noticed similar perforations in 2 out of 15 patients (13%).
Though Stock et al.18 have not reported the perioperative complications and late toxicity in their series, we noticed a minimal perioperatve (after the removal of implant) complication rate (2.5%) in the form of hematuria. This was not because of the misplacement of needle in bladder during implant procedure as the post-procedure CT scan (done for planning) did not show any needle in the bladder. This could have been due to accidental movement of needle during shifting of the patient in the brachytherapy suite or in the indoor unit where the implant was removed. Similarly, late toxicity rate of 12% in our study was also low and comparable to most studies in the literature using IBT.2 Group III patients in our series had higher late toxicity, since they already had received previous RT for the initial treatment. Due to short follow up and heterogeneous patient population in our study, we could not calculate the survival but the local pelvic control in our series is comparable to other studies.
There is no standard dose fractionation schedule of HDR IBT for cervical carcinoma. American Brachytherapy Society in its guidelines25 suggests 2 to 3 sessions of weekly HDR IBT, each session delivering 2 fractions of 5.5 to 6.0 Gy each. We used 2 sessions (one session per week), each session delivering a dose of 10 Gy in a single fraction. The rationale of using high dose per fraction is the short treatment time, equal effectiveness, convenient and least morbid. Such an HDR dose fractionation schedule has already been tried for ICRT by Patel et al.26 They have used a dose of 9 Gy to point A in 2 weekly fractions and reported successful clinical results. Therefore an HDR dose of 8 to 10 Gy is biologically equivalent and clinically effective dose. Additionally, with single high dose of 10 Gy (un-fractionated), the treatment is completed within 2 to 3 hours which has several advantages like least trauma, least probability of bleeding, infection, fistulas etc. At the same time, it is highly convenient and comfortable to patient.
A few studies7,8,10 have mentioned the use of TAUS in some proportion of their patients, but no details are reported. Though TAUS is simple and relatively non-invasive, the image quality is compromised due to abdominal wall thickness, bowel gas etc. Various other imaging techniques described so far in the literature for cervical cancer IBT, have individual limitations. Some of them like CT/MRI scan provide better images but are not practical in the operative room. Xray/fluoroscopy are simple easily available, but do not image the normal pelvic structures or the tumor area. Laparoscopy, lapartomy guided implants are restricted to only of few centers and have been tried very anecdotally. Comparatively, TRUS is simple and convenient technique and provide real time images of the IBT needles, normal structures and to some extent the tumor.
Our series of 40 TRUS guided IBT procedures in 25 patients with cervical cancer, suggests that TRUS assists in avoiding the needle injury/perforation of pelvic structures very accurately and precisely thereby reducing the risk of perioperative complications. It has many advantages as compared to other imaging techniques tried so far. To conclude, TRUS is most practical and effective imaging device for guiding the IBT procedure for cervical cancer patients. It is perhaps the ideal choice for developing countries where cervical cancer is the commonest cancer in females and the resources are limited.
Figures and Tables
Fig. 1
Transrectal ultrasound axial image showing the cervical tumor region with central tandem in the cervical os.
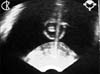
Fig. 2
Longitudinal image demonstrating the stainless steel brachytherapy needle (bright shadow) which is about to pierce the gut wall.

Fig. 3
Transrectal ultrasound image showing the brachytherapy needles in the cervical tumor region (dotted line) covering it adequately.

Fig. 4
Isodose distribution of the patient created by CT based planning. Thick red line depicts the target area obtained by line joining the outermost needles. The thin red line represents the prescription isodose (100% isodose line).
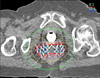
Table 2
Details of the brachytherapy implant procedures performed under the transrectal ultrasound (TRUS) guidance

References
1. Perez CA, Kavanagh BD. Halperin EC, Parez CA, Brady LW, editors. Uterine cervix. Perez and Brady's principles and practice of radiation oncology. 2008. 5th ed. Philadelphia: Lippincott Williams & Wilkins;1532–1609.
2. Erickson B, Gillin MT. Nag S, editor. Interstitial implantation of gynecological malignancies. Principles and practice of brachytherapy. 1997. Armonk: Futura Publishing Company;515–552.
3. Martinez A, Herstein P, Portnuff J. Interstitial therapy of perineal and gynecological malignancies. Int J Radiat Oncol Biol Phys. 1983. 9:409–416.
4. Martinez A, Cox RS, Edmundson GK. A multiple-site perineal applicator (MUPIT) for treatment of prostatic, anorectal, and gynecologic malignancies. Int J Radiat Oncol Biol Phys. 1984. 10:297–305.
5. Syed AMN, Puthawala AA, Neblett D, Disaia PJ, Berman ML, Rettennaier M, et al. Transperineal interstitial-intracavitary "Syed-Neblett" applicator in the treatment of carcinoma of the uterine cervix. Endocurie Hypertherm Oncol. 1986. 2:1–13.
6. Erickson B, Albano K, Gillin M. CT-guided interstitial implantation of gynecologic malignancies. Int J Radiat Oncol Biol Phys. 1996. 36:699–709.
7. Demanes DJ, Rodriguez RR, Bendre DD, Ewing TL. High dose rate transperineal interstitial brachytherapy for cervical cancer: high pelvic control and low complication rates. Int J Radiat Oncol Biol Phys. 1999. 45:105–112.
8. Beriwal S, Bhatnagar A, Heron DE, Selvaraj R, Mogus R, Kim H, et al. High-dose-rate interstitial brachytherapy for gynecologic malignancies. Brachytherapy. 2006. 5:218–222.
9. Nag S, Martinez-Monge R, Ellis R, Lewandowski G, Vacarello L, Boutselis JG, et al. The use of fluoroscopy to guide needle placement in interstitial gynecological brachytherapy. Int J Radiat Oncol Biol Phys. 1998. 40:415–420.
10. Erickson B, Foley WD, Gillin M, Albano K, Wilson JF. Ultrasound-guided transperineal interstitial implantation of pelvic malignancies: description of the technique. Endocurie Hypertherm Oncol. 1995. 11:107–113.
11. Corn BW, Lanciano RM, Rosenblum N, Schnall M, King S, Epperson R. Improved treatment planning for the Syed-Neblett template using endorectal-coil magnetic resonance and intraoperative (laparotomy/laparoscopy) guidance: a new integrated technique for hysterectomized women with vaginal tumors. Gynecol Oncol. 1995. 56:255–261.
12. Viswanathan A, Cormack R, Holloway CL, Tanaka C, O'Farrell D, Devlin PM, et al. Magnetic resonance-guided interstitial therapy for vaginal recurrence of endometrial cancer. Int J Radiat Oncol Biol Phys. 2006. 66:91–99.
13. Recio FO, Piver MS, Hempling RE, Eltabbakh GH, Hahn S. Laparoscopic-assisted application of interstitial brachytherapy for locally advanced cervical carcinoma: results of a pilot study. Int J Radiat Oncol Biol Phys. 1998. 40:411–414.
14. Monk BJ, Walker JL, Tewari K, Ramsinghani NS, Nisar Syed AM, DiSaia PJ. Open interstitial brachytherapy for the treatment of local-regional recurrences of uterine corpus and cervix cancer after primary surgery. Gynecol Oncol. 1994. 52:222–228.
15. Fleischer AC, Burnett LS, Murray MJ, Jones HW 3rd. Intraoperative guidance for intrauterine procedures with transrectal sonography. Radiology. 1990. 176:576–577.
16. Holm HH, Juul N, Pedersen JF, Hansen H, Stroyer I. Transperineal 125iodine seed implantation in prostatic cancer guided by transrectal ultrasonography. J Urol. 1983. 130:283–286.
17. Kini VR, Edmundson GK, Vicini FA, Jaffray DA, Gustafson G, Martinez AA. Use of three-dimensional radiation therapy planning tools and intraoperative ultrasound to evaluate high dose rate prostate brachytherapy implants. Int J Radiat Oncol Biol Phys. 1999. 43:571–578.
18. Stock RG, Chan K, Terk M, Dewyngaert JK, Stone NN, Dottino P. A new technique for performing Syed-Neblett template interstitial implants for gynecologic malignancies using transrectal-ultrasound guidance. Int J Radiat Oncol Biol Phys. 1997. 37:819–825.
19. Haie-Meder C, Potter R, Van Limbergen E, Briot E, De Brabandere M, Dimopoulos J, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005. 74:235–245.
20. Potter R, Haie-Meder C, Van Limbergen E, Barillot I, De Brabandere M, Dimopoulos J, et al. Recommendations from Gynaecological (GYN) GEC ESTRO Working Group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006. 78:67–77.
21. Brascho DJ, Kim RY, Wilson EE. Use of ultrasonography in planning intracavitary radiotherapy of endometrial carcinoma. Radiology. 1978. 129:163–167.
22. Granal CO, Allee P, Doherty F, Madoc-Jones H, Curry SL. Ultrasound used for assessing the in situ position of intrauterine tandems. Gynecol Oncol. 1984. 18:334–338.
23. Granai CO, Allee P, Doherty F, Ball HG, Madoc-Jones H, Curry SL. Intraoperative real-time ultrasonography during intrauterine tandem placement. Obstet Gynecol. 1986. 67:112–114.
24. Rotmensch J, Waggoner SE, Quiet C. Ultrasound guidance for placement of difficult intracavitary implants. Gynecol Oncol. 1994. 54:159–162.
25. Nag S, Erickson B, Thomadsen B, Orton C, Demanes JD, Petereit D. The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2000. 48:201–211.
26. Patel FD, Sharma SC, Negi PS, Ghoshal S, Gupta BD. Low dose rate vs. high dose rate brachytherapy in the treatment of carcinoma of the uterine cervix: a clinical trial. Int J Radiat Oncol Biol Phys. 1994. 28:335–341.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download