Abstract
Objective
Wine has been the focus in the prevention of epithelial ovarian cancer (EOC) development because resveratrol abundant in wine has anti-carcinogenic properties. However, epidemiologic results have been heterogenous in the chemopreventive effect of wine on the development of EOC. Thus, we performed a meta-analysis for comparing EOC risk between wine and never drinkers using previous related studies.
Methods
After extensive search of the literature between January 1986 and December 2008, we analyzed 10 studies (3 cohort and 7 case control studies) with 135,871 women, who included 65,578 of wine and 70,293 of never drinkers.
Results
In all studies, there was no significant difference in EOC risk between wine and never drinkers (odds ratio [OR], 1.13; 95% confidence interval [CI], 0.92 to 1.38; random effects). When we performed re-analysis according to the study design, 3 cohort and 7 case control studies showed that there were also no significant differences in EOC risk between wine and never drinkers, respectively (OR, 1.44 and 1.04; 95% CI, 0.74 and 2.82 and 0.88 to 1.22; random effects). In sub-analyses using 2 case-control studies, EOC risk was not different between former and never drinkers (OR, 1.12; 95% CI, 0.87 to 1.44; fixed effect), and between current and former drinkers (OR, 0.74; 95% CI, 0.41 to 1.34; random effects).
Ovarian cancer is the second most common malignancy of female genital tract in the world,1 and the incidence has increased to about 25% of gynecologic cancers in Korea.2 Epithelial ovarian cancer (EOC), which accounts for about 90% of ovarian cancer, is diagnosed as advanced-stage disease in more than two-thirds of all patients because of vague symptoms and no effective screening methods.3 Moreover, as many as 80% of patients with advanced-stage disease ultimately relapse albeit the primary standard treatment made up of maximal cytoreductive surgery followed by adjuvant taxane- and platinum-based chemotherapy.4 Although molecular targeted therapies, the majority of which are small-molecule inhibitors or monoclonal antibodies, are attractive treatment options due to the cytostatic effect against tumors, more preclinical and clinical studies are needed to evaluate the efficacy and toxicity for the treatment of EOC.5
Thus, the management of EOC has been extended to the concept of "chemoprevention", wherein naturally occurring or synthetic chemical agents from dietary factors are used as phytochemicals for the inhibition, delay or even reversal of ovarian carcinogenesis.6 Among the dietary factors, wine has been focused on with regard to the chemoprevention of EOC because it is abundant in various antioxidants and also contains resveratrol, a phytoestrogen with anti-carcinogenic properties in recent preclinical studies.7-11 However, several epidemiologic results have been heterogenous regarding the association between wine drinking and EOC risk.11-29 Therefore, the current study was designed to evaluate the efficacy of wine drinking for reducing EOC risk through a meta-analysis using previous relevant studies.
A literature search of the National Library of Medicine and National Institutes of Health (PubMed), EMBASE and Cochrane Controlled Trials Register (CENTRAL) electronic database was performed independently by 2 reviewers for this meta-analysis. The literature search was limited to the time period between January 1986 and December 2008. We also searched the bibliographies of relevant articles for indentifying additional studies.
We performed the computerized literature search using the free text search terms "ovarian cancer," "ovarian neoplasm," "ovarian tumor," "ovarian carcinoma," "wine," "red wine," "white wine" for the outcome factors. All terms were expanded to include all sub-categories in an attempt to obtain all published research that fit the selection criteria. No financial conflict of interest existed with any commercial entity whose products are described, reviewed, evaluated or compared in this meta-analysis.
To be enrolled in the current study, retrieved studies had to fulfill the following inclusion criteria: 1) EOC; 2) comparison of the incidence of EOC between wine and never drinkers. Exclusion criteria included: 1) non-EOC; 2) insufficient data about wine consumption; 3) lack of accessibility to original articles. All resulting citation abstracts were reviewed for potential eligibility, and the full article texts were obtained for further evaluation in cases that the abstracts did not provide enough details for the determination of eligibility.
A total of 19 potentially relevant studies were identified based on the above search terms, and all of the retrieved studies were independently evaluated. After screening the abstracts, 4 studies were excluded because of irrelevance, including other diseases (n=2),22,23 and basic research (n=2).11,24 Further assessment for more detailed information identified 2 ineligible studies because duplication (n=1),25 and reply to an original article (n=1).26 After we reviewed full manuscripts of the remaining studies, 3 studies were excluded because we could not obtain relevant data for this meta-analysis in spite of the request to related authors.27-29 Finally, 7 case-controls and 3 cohort studies were scrutinized in full text as appropriate (Fig. 1).12-21
The following data were independently abstracted for this meta-analysis: first author; year of publication; age; duration of enrollment and follow-up; geographic location; study design; numbers of cases (wine drinker) and controls (never drinker); exposure level to wine; factors for adjustment. Two reviewers compared the results of abstraction from all 10 studies for accuracy and came to an agreement on any discrepancies. In one study with disagreement, a third reviewer served as the tiebreaker.14
The aim of this meta-analysis was to compare the incidence of EOC between wine and never drinkers. In the current study, "never drinker" was defined as a person who had never drunk wine, whereas "wine drinker" included "current drinker (a person who drinks wine currently)" or "former drinker (a person who have not imbibed for a year or more; a person who has stopped drinking wine for more than 9 months)." For sub-analyses, we compared the incidence of EOC 1) between current and former drinkers and 2) between former and never drinkers.
The dichotomous data eligible for this meta-analysis in each study were expressed as an odds ratio (OR) with 95% confidence interval (CI). These results were combined with use of the Mantel-Haenszel method when using the fixed effect model, and the DerSimonian and Laird method when using the random effects model.
Heterogeneity was assessed using Higgins I2, which measures the percentage of the total variation across studies that is due to heterogeneity rather than chance. I2 is evaluated as follows: I2=(Q-df)/Q×100%, where Q is Cochran's heterogeneity statistic and df is its degrees of freedom. The value of I2 ranges from 0% (no observed heterogeneity) to 100% (maximal heterogeneity). An I2 >50% may be considered to represent substantial heterogeneity.30
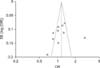
For identifying publication bias, a funnel plot was represented, which is a scatter plot of ORs of enrolled studies on the X-axis against the standard error of log OR of each study on the Y-axis. If there is no publication bias, ORs of small-scale studies scatter widely at the bottom of the graph, with the spread narrowing among large-scale studies. The funnel plot resembles a symmetrical inverter funnel in the absence of publication bias, whereas publication bias makes the funnel plot asymmetrical.31
This meta-analysis was performed using Review Manager ver. 5.0 (The Nordic Cochrane Centre, Copenhagen, Denmark). The fixed effects model was used when heterogeneity was not present, whereas we applied the random effects model in the presence of significant heterogeneity. A p-value of < 0.05 was considered to be statistically significant.
Clinical characteristics of a total of 135,871 women from 10 relevant studies are depicted in Table 1. Among all, 5,568 women (4.1%) had EOC, and 65,578 (48.3%) and 70,293 (51.7%) were wine and never drinkers, respectively. When we analyzed EOC risk between wine and never drinkers, there was no significant difference in EOC risk between wine and never drinkers (OR, 1.14; 95% CI, 0.91 to 1.43; I2=88%). When we performed re-analysis according to the study design, the random effects models using 3 cohort and 7 case-control studies demonstrated that there was also no significant difference in EOC risk between wine and never drinkers, respectively (OR, 1.44 and 1.04; 95% CI, 0.74 to 2.82 and 0.88 to 1.22; I2=95% and 76%) (Fig. 2).
Among all studies, 2 enabled sub-analyses where we compared EOC risk 1) between former and never drinkers, and 2) between current and former drinkers.18,19 As a result, EOC risk was not different between former and never drinkers (OR, 1.12; 95% CI, 0.87 to 1.44; I2=0%), and between current and former drinkers (OR, 0.74; 95% CI, 0.41 to 1.34; I2=74%) (Fig. 3).
Tests for heterogeneity showed that there was significant between-study variation (I2=74% to 95%) except for 1 sub-analysis (I2=0%). However, the funnel plot for 10 eligible studies revealed that all studies were distributed evenly across the graph, suggesting no publication bias in this meta-analysis (Fig. 4).
Alcohol consumption may influence EOC risk through the effects on steroid hormones, especially estrogens, which are believed to play a primary role in ovarian carcinogenesis.32 Mechanisms of alcohol-related ovarian carcinogenesis include increased cumulative estrogen exposure, alteration of gonadotropin levels, promotion of DNA damage, impaired folate metabolism, DNA hypomethylation, inhibition of carcinogen detoxification or clearance, and increased metastatic potential of tumor cells.33,34 On the other hand, resveratrol abundant in wine has emerged as one of the most promising naturally occurring compound with chemopreventive potential. Resveratrol is a trans-3, 5, 4'-trihydroxystilbene highly abundant in grapes, moderately abundant in blueberries, peanuts and sparsely abundant in many other plants.6 Resveratrol has a number of naturally occurring analogs such as pterostilbene, piceatannol and oxyresveratrol, which may have anti-inflammatory, anti-carcinogenic, cell cycle inhibitory and anti-oxidant effects.35-38 Since wine contains higher levels of resveratrol than spirits and beer, it is plausible that wine may influence EOC risk independently of the alcohol that it contains.
Some epidemiologic studies have shown that high levels of resveratrol found in wine may reduce the risk of EOC,18,20,25 suggesting the strong protective effect of resveratrol in wine on the development of EOC. On the other hand, other studies have reported the significant positive association between wine consumption and EOC risk,14,16,19 emphasizing that total amount of alcohol is more important than the chemopreventive effect of wine on the development of EOC.
Nevertheless, our results showed that there was no association between wine drinking and EOC risk (OR, 1.14; 95% CI, 0.91 to 1.43) that has been shown in a previous study.27 This fact was not altered when we performed the re-analysis using 3 cohort and 7 case-control studies (OR, 1.44 and 1.04; 95% CI, 0.74 to 2.82 and 0.88 to 1.22). Moreover, former or current consumption of wine was not associated with EOC risk compared with never drinking of wine (OR, 1.12 and 0.74; 95% CI, 0.87 to 1.44 and 0.41 to 1.34). The reason is that phytochemicals including resveratrol in wine have multifarious effects including pro-estrogenic activity and possible genotoxicity, albeit their anti-proliferative and antioxidant properties.39,40 It means that both potentially beneficial and harmful effects of phytochemicals in wine should be considered together in the development of EOC.
However, this meta analysis should be interpreted in light of some limitations: first, we could not distinguish red from white wine consumption, even though high concentrations of resveratrol is known to be mainly in red wine6; second, we were not able to obtain data about consumption of other alcoholic beverages, and drinkers of other alcoholic beverages may be also regarded as never drinkers of wine in this meta analysis. Moreover, insufficient data from 3 relevant may be biased in this meta-analysis.27-29 Thus, these facts may act as confounding factors for comparing EOC risk between wine and never drinkers; Third, we could not evaluate the chemopreventive effect of wine according to dose-dependency because cut-off levels of the increased wine consumption were different among all 7 studies. Fourth, we could not also evaluate EOC risk according to menopausal status and histological types because of the lack of related data, and the results of this meta-analysis should be interpreted considering different covariates for risk estimation.
Conclusively, wine, especially resveratrol, will be focused on increasingly as a phytochemical with chemopreventive potential on the development of EOC in the future. Nonetheless, the precise effect and mode of action of resveratrol has remained enigmatic, and epidemiologic results cannot support the chemopreventive effect of wine on EOC risk because of many limitations including study design and confounding factors. Therefore, more rigorous preclinical and clinical evaluation of its chemopreventive effect will further delineate its true potential for reducing the development of EOC.
Figures and Tables
Fig. 3
Comparison of epithelial ovarian cancer risk between former and never drinkers, and between current and former drinkers.

ACKNOWLEDGEMENT
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2009-0083687).
References
1. Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006. 20:207–225.
2. Kim K, Ryu SY. Major clinical research advances in gynecologic cancer 2009. J Gynecol Oncol. 2009. 20:203–209.
3. Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, et al. Carcinoma of the ovary: FIGO 6th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006. 95:Suppl 1. S161–S192.
4. Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003. 21:3194–3200.
5. Blagden S, Gabra H. Promising molecular targets in ovarian cancer. Curr Opin Oncol. 2009. 21:412–419.
6. Goswami SK, Das DK. Resveratrol and chemoprevention. Cancer Lett. 2009. 284:1–6.
7. Raj MH, Abd Elmageed ZY, Zhou J, Gaur RL, Nguyen L, Azam GA, et al. Synergistic action of dietary phyto-antioxidants on survival and proliferation of ovarian cancer cells. Gynecol Oncol. 2008. 110:432–438.
8. Park SY, Jeong KJ, Lee J, Yoon DS, Choi WS, Kim YK, et al. Hypoxia enhances LPA-induced HIF-1alpha and VEGF expression: their inhibition by resveratrol. Cancer Lett. 2007. 258:63–69.
9. Kueck A, Opipari AW Jr, Griffith KA, Tan L, Choi M, Huang J, et al. Resveratrol inhibits glucose metabolism in human ovarian cancer cells. Gynecol Oncol. 2007. 107:450–457.
10. Tyagi A, Singh RP, Agarwal C, Siriwardana S, Sclafani RA, Agarwal R. Resveratrol causes Cdc2-tyr15 phosphorylation via ATM/ATR-Chk1/2-Cdc25C pathway as a central mechanism for S phase arrest in human ovarian carcinoma Ovcar-3 cells. Carcinogenesis. 2005. 26:1978–1987.
11. Opipari AW Jr, Tan L, Boitano AE, Sorenson DR, Aurora A, Liu JR. Resveratrol-induced autophagocytosis in ovarian cancer cells. Cancer Res. 2004. 64:696–703.
12. Larsson SC, Giovannucci E, Wolk A. Dietary folate intake and incidence of ovarian cancer: the Swedish Mammography Cohort. J Natl Cancer Inst. 2004. 96:396–402.
13. Schouten LJ, Zeegers MP, Goldbohm RA, van den Brandt PA. Alcohol and ovarian cancer risk: results from the Netherlands Cohort Study. Cancer Causes Control. 2004. 15:201–209.
14. Chang ET, Canchola AJ, Lee VS, Clarke CA, Purdie DM, Reynolds P, et al. Wine and other alcohol consumption and risk of ovarian cancer in the California Teachers Study cohort. Cancer Causes Control. 2007. 18:91–103.
15. Gwinn ML, Webster LA, Lee NC, Layde PM, Rubin GL. Alcohol consumption and ovarian cancer risk. Am J Epidemiol. 1986. 123:759–766.
16. La Vecchia C, Negri E, Franceschi S, Parazzini F, Gentile A, Fasoli M. Alcohol and epithelial ovarian cancer. J Clin Epidemiol. 1992. 45:1025–1030.
17. Tavani A, Gallus S, Dal Maso L, Franceschi S, Montella M, Conti E, et al. Coffee and alcohol intake and risk of ovarian cancer: an Italian case-control study. Nutr Cancer. 2001. 39:29–34.
18. Goodman MT, Tung KH. Alcohol consumption and the risk of borderline and invasive ovarian cancer. Obstet Gynecol. 2003. 101:1221–1228.
19. Modugno F, Ness RB, Allen GO. Alcohol consumption and the risk of mucinous and nonmucinous epithelial ovarian cancer. Obstet Gynecol. 2003. 102:1336–1343.
20. Webb PM, Purdie DM, Bain CJ, Green AC. Alcohol, wine, and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2004. 13:592–599.
21. Peterson NB, Trentham-Dietz A, Newcomb PA, Chen Z, Hampton JM, Willett WC, et al. Alcohol consumption and ovarian cancer risk in a population-based case-control study. Int J Cancer. 2006. 119:2423–2427.
22. Rosenberg L, Slone D, Shapiro S, Kaufman DW, Helmrich SP, Miettinen OS, et al. Breast cancer and alcoholic-beverage consumption. Lancet. 1982. 1:267–270.
23. Celik Y, Temizoz O, Genchellac H, Cakir B, Asil T. A non-alcoholic patient with acute Marchiafava-Bignami disease associated with gynecologic malignancy: paraneoplastic Marchiafava-Bignami disease? Clin Neurol Neurosurg. 2007. 109:505–508.
24. Yang SH, Kim JS, Oh TJ, Kim MS, Lee SW, Woo SK, et al. Genome-scale analysis of resveratrol-induced gene expresssion profile in human ovarian cancer cells using a cDNA microarray. Int J Oncol. 2003. 22:741–750.
25. Larsson SC, Wolk A. Wine consumption and epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2004. 13:1823.
26. Gallus S, Scotti L, Talamini R, Franceschi S, Dal Maso L, Negri E, et al. Reply to: alcohol consumption and ovarian cancer risk in a population-based case-control study by Peterson et al. Int J Cancer. 2007. 121:2578–2579.
27. Genkinger JM, Hunter DJ, Spiegelman D, Anderson KE, Buring JE, Freudenheim JL, et al. Alcohol intake and ovarian cancer risk: a pooled analysis of 10 cohort studies. Br J Cancer. 2006. 94:757–762.
28. Tworoger SS, Gertig DM, Gates MA, Hecht JL, Hankinson SE. Caffeine, alcohol, smoking, and the risk of incident epithelial ovarian cancer. Cancer. 2008. 112:1169–1177.
29. Chang ET, Lee VS, Canchola AJ, Dalvi TB, Clarke CA, Reynolds P, et al. Dietary patterns and risk of ovarian cancer in the California Teachers Study cohort. Nutr Cancer. 2008. 60:285–291.
30. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003. 327:557–560.
31. Sterne JA, Egger M. Funnel plots for detecting bias in meta analysis: guidelines on choice of axis. J Clin Epidemiol. 2001. 54:1046–1055.
32. Ho SM. Estrogen, progesterone and epithelial ovarian cancer. Reprod Biol Endocrinol. 2003. 1:73.
33. Singletary KW, Gapstur SM. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA. 2001. 286:2143–2151.
34. Cooper GS, Sandler DP, Whelan EA, Smith KR. Association of physical and behavioral characteristics with menstrual cycle patterns in women age 29-31 years. Epidemiology. 1996. 7:624–628.
35. Rimando AM, Cuendet M, Desmarchelier C, Mehta RG, Pezzuto JM, Duke SO. Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. J Agric Food Chem. 2002. 50:3453–3457.
36. Wolter F, Clausnitzer A, Akoglu B, Stein J. Piceatannol, a natural analog of resveratrol, inhibits progression through the S phase of the cell cycle in colorectal cancer cell lines. J Nutr. 2002. 132:298–302.
37. Potter GA, Patterson LH, Wanogho E, Perry PJ, Butler PC, Ijaz T, et al. The cancer preventative agent resveratrol is converted to the anticancer agent piceatannol by the cytochrome P450 enzyme CYP1B1. Br J Cancer. 2002. 86:774–778.
38. Shin NH, Ryu SY, Choi EJ, Kang SH, Chang IM, Min KR, et al. Oxyresveratrol as the potent inhibitor on dopa oxidase activity of mushroom tyrosinase. Biochem Biophys Res Commun. 1998. 243:801–803.
39. Wietrzyk J, Grynkiewicz G, Opolski A. Phytoestrogens in cancer prevention and therapy: mechanisms of their biological activity. Anticancer Res. 2005. 25:2357–2366.
40. Stopper H, Schmitt E, Kobras K. Genotoxicity of phytoestrogens. Mutat Res. 2005. 574:139–155.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download