Abstract
Objective
The aim of this study was to determine whether the presence of bacterial vaginosis (BV) is associated with cervical intraepithelial neoplasia (CIN) and human papilloma virus (HPV) infection.
Methods
A total of 588 women who had abnormal Pap smears and had finally undergone loop electrosurgical excision procedure (LEEP) in our institute from September 2002 to May 2006 were selected. The screening tests for BV were done in 552 of the 588, and BV was diagnosed if three of the following four findings were satisfied: presence of abnormal discharge, vaginal pH>4.5, presence of clue cells, positive amine or whiff test. Five hundred and five patients had HPV typing tests by the HPV DNA chip. Forty two patients diagnosed with invasive cancer were excluded from this study. CIN was subdivided into low-grade CIN (CIN I) and high-grade CIN (CIN II/III) groups.
Results
There was no statistically significant difference in patient characteristics between BV-present and BV-absent group. The incidence of CIN was significantly higher in the BV-present group (p=0.043), however, no statistical significance of BV on CIN was observed on multivariate analysis. HPV infection showed no significant relationship with BV. BV with or without HPV infection did not influence the incidence of CIN, regardless of the severity.
There are numerous risk factors for cervical intraepithelial neoplasia (CIN) and cervical cancer, such as young age at first intercourse (<16 years), multiple sexual partners, cigarette smoking, race, high parity, low socioeconomic status. Recently, it was demonstrated that the initiating event in cervical dysplasia and carcinogenesis is likely to be infected with human papilloma virus (HPV). However, most women who are infected with HPV have no apparent clinical evidence of disease. Cervical cancer develops only in a small number of women with HPV infection. Moreover, pretreatment HPV viral loads do not correlate with prognostic risk factors.1 It is suggested that additional cofactors partake in cervical carcinogenesis. Factors that may have a role in this progression include smoking, contraceptive use, nutrition and infection with sexually transmitted diseases (STDs), such as bacterial vaginosis (BV), chlamydia trachomatis and trichomonas vaginalis.2,3 As the abnormal vaginal flora can produce carcinogenic nitrosamines and BV is similar with CIN in epidemiologic features, we hypothesized that BV might have an important role in the development of CIN.4 There have been several studies on this subject, but their results were not consistent.
We subdivided the study groups into BV present and BV absent groups and compared the incidence of CIN.
Therefore, the aim of this study was to clarify the association of BV with CIN and HPV infection among Korean women who had undergone loop electrosurgical excision procedure (LEEP).
Women referred to our institute from September 2002 to May 2006 for investigation of abnormal cervical cytology on the Papanicolaou (PAP) test were enrolled in this study. All patients were submitted to thin-prep PAP test, colposcopy and directed biopsy, and endocervical curettage. HPV test was done in the patients with no evaluating history. For STD screening, vaginal swabs for trichomonas vaginalis and candida species, polymerase chain reaction (PCR) for Chlamydia trachomatis and cervical cultures were performed. Pregnant or menstruating patient at the time of examination were excluded from this study. They were not treated with antibiotics or subject to surgical procedures before examination.
BV is a common, treatable condition resulting from replacement of the normal hydrogen peroxide-producing Lactobacillus-predominant vaginal flora with anaerobic bacteria, e.g. Gardnella vaginalis, Mobiluncus species and Mycoplasma hominis.5 Factors that increase the risk of BV are cigarette smoking, the use of intrauterine devices, frequent douches, multiple sexual partners, and early age at first intercourse. BV is known to be associated with many obstetric and gynecologic complications such as preterm labor and delivery, chorioamnionitis, post-cesarean endometritis, pelvic inflammatory disease (PID), postabortal PID, postoperative cuff infections after hysterectomy, and a possible connection with abnormal cervical cytology and CIN.6-10
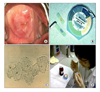
BV was diagnosed if three of the following four findings were satisfied: presence of thin, grey vaginal secretions coating the vaginal wall; vaginal pH>4.5; presence of clue cell on microscopic examination of vaginal smear; positive amine or whiff test (Fig. 1).11
The result of PAP smears was reported according to the Bethesda III system (2001): atypical squamous cell (ASC), low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL). LSIL includes CIN 1 and koilocytotic atypia. HSIL includes CIN 2 and CIN 3. The result of cervical biopsies was reported according to the CIN classification system as mild (CIN 1), moderate (CIN 2), or severe (CIN 3) dysplasia or carcinoma in situ (CIS).12 CIN was subdivided into a low-grade CIN (CIN I) group and a high-grade CIN (CIN II/III) group.
HPV detection and genotyping was performed with HPV DNA Chip, a PCR-based DNA microarray system provided by Microarray Center, Biomedlab Co (Seoul, Korea). HPV DNA Chip contains 22 type-specific probes that consist of 15 high-risk groups (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 69) and 7 low-risk groups (6, 11, 34, 40, 42, 43, and 44). DNA was isolated from swab samples with a DNA isolation kit (Qiagen, Hilden, Germany), and target HPV DNA was amplified by PCR with GPd5+/Gp6d+ primers (GP5d+, 5'-tttkttachgtkgtdgatacyac-3'; GP6d+, 5'-gaaahataaaytgyaadtcataytc-3'; k, g/ t; h, t/a/c; d, a/t/g; y, t/c). β-Globin was PCR amplified with PC03/PC04 primers (PC03, 5'-acacaactgtgttcactagc-3'; PC04, 5'-caacttcatccacgttcacc-3') as an internal control. Amplified DNA was labeled by Cy5-dUTP (NEN; Life Science Products, Inc, Boston, MA, USA). A mixture of 10 HPV-amplified products and 5 β-globin-amplified products were denatured by the addition of 3N sodium hydroxide solution (10% vol/vol), followed by incubation for 5 minutes at room temperature, were neutralized by the addition of 1 mol/L TRIS (tris-[hydroxymethyl]-aminoethane)-hydrochloric acid (pH, 7.2; 5% vol/vol) then 3N hydrochloric acid (10% vol/vol), and finally being cooled for 5 minutes on ice. The samples were mixed with a hybridization solution made of 6_SSPE (saline-sodium phosphate-EDTA buffer; Sigma Chemical Co, St Louis, MO, USA) and 0.2% sodium dodecylsulfate and applied to the DNA chip. The hybridization was performed at 40℃ for 2 hours and then washed with 3_SSPE for 2 minutes, 1_SSPE for 2 minutes, and air-dried at room temperature. Hybridized HPV DNA was visualized with the use of a DNA Chip Scanner (Scanarray lite; GSI Lumonics, Ottawa, Canada).13
LEEP was performed in women with high grade CIN or with a discrepancy between cytologic and histologic results. Statistical analysis was performed by the student t-test, chi-square test and Fisher's exact test (when n was less than 5), and logistic regression analysis with SPSS ver. 12.0. All statistical tests were two-sided. A level of p<0.05 was accepted as statistically significant.
Among the 588 patients who underwent LEEP, 552 patients had screening tests for diagnosing BV, and among them 505 patients had HPV typing tests by HPV DNA chip. A total of 510 patients, excluding 42 patients diagnosed with invasive cancer, were included in this study. Fifty six patients (0.98%) were positive for BV and 454 patients (99.02%) were negative for BV. There were no significant differences in baseline clinical and demographic characteristics between the two groups (Table 1).
HPV testing of cervical cells was performed for 505 out of 588 patients, and 473 out of 510 patients in this study. Among 473 patients, 327 patients (69.1%) were positive for HPV infection. HPV was present in 41 patients (77.4%) with BV, and in 286 patients (68.1%) without BV. Table 1 also shows that there was no significant correlation between the presence of BV and HPV infection (p=0.196).
Comparison of the incidence of CIN (confirmed either by histology or by colposcopically directed biopsy or by LEEP) according to the presence of BV was performed (Table 2). Among the 454 patients without BV, CIN was diagnosed in 384 patients (81.8%). Among the 56 patients with BV, CIN was diagnosed in 53 patients (94.6%). The incidence of CIN was significantly higher in the BV-present group (p=0.043), but the multivariate analysis using logistic regression analysis revealed no statistical significance (p=0.081). The presence of BV was not associated with the severity of CIN (p=0.777). When CIN patients were subdivided into low-grade CIN (CIN I) group and high-grade CIN (CIN II/III) groups, low-grade CIN was present in 181 patients and high-grade CIN was present in 256 patients. Twenty one patients with BV had lowgrade CIN and 32 patients (12.5%) had high-grade CIN (Table 3).
HPV infection in this study also showed strong positive correlation with the incidence of CIN (p≤0.001) (Table 4).
CIN is known to be related to numerous epidemiological factors such as first intercourse at young age (<16 years), multiple sexual partners, cigarette smoking, race, high parity, and low socioeconomic status. Many of these epidemiological factors are linked with sexual activity and with exposure to STDs. However, the pathogenesis of BV still remains unclear.
As for the association between BV and HPV infection, this study showed that HPV infection had no significant relationship with BV (p=0.873) (Table 1), but showed strong positive correlation with the incidence of CIN (p≤0.001) (Table 4).
Platz-Christensens et al.14 demonstrated the association between BV and CIN. For the diagnosis of BV, they used identification of clue cells in Papanicolaou stained vaginal smears. They showed that the relative risk for having CIN III/CIS was 5.0 if BV was present. However, this study did not include a control for the presence of HPV nor other STDs. Peters et al. reported a similar study on the association between BV and CIN.15 They used Amsel's criteria for BV diagnosis and performed control for HPV infection. This study concluded that in women with abnormal cervical smears, the prevalence of BV did not seem to be increased, and BV did not influence the histologic changes. There was no relationship between BV and HPV infection. These studies revealed different outcomes from one another probably due to the difference in their methods. Since CIN carries a variety of risk factors, it is important to control them between the study groups - especially the status of HPV infection which is known as a major risk factor of CIN. Meanwhile, the sensitivity and specificity of BV differ according to several different diagnostic criteria. Therefore, we should allow for the various diagnostic criteria applied to each study. Especially for clinical diagnosis, various diagnostic tools and interpretation skills are required, but bias may occur due to subjective opinions of clinicians. Furthermore, those factors such as coitus, douching, and cervical mucus may interfere with the diagnosis. Due to the reasons mentioned above, clinical diagnostic methods may result in different diagnosis according to different clinicians even when they use the same diagnostic criteria.
Boyle et al.16 reported that women with BV were not found to have CIN more frequently than women without BV. Furthermore, they demonstrated that the quantities of nitrosamines produced by women with BV did not differ significantly from women without BV. Chen et al.17 reported that abnormal amines are closely related to the presence of BV, and can be eliminated by treatment with metronidazole. Pavic proposed a theory regarding the oncogenic effect of nitrosamines, and also discussed the possibility that nitrosamines may act synergistically with other agent such as HPV.4,18 However, according to recent studies, these results are questionable.
In this study, BV was found in 56 (10.98%) out of 510 patients. In other studies, its prevalence varies; 32 to 64% in STD clinics, 12 to 25% in gynecology outpatient clinics, 10 to 26% in antenatal clinics.4,19-21 The low incidence of BV in this study must be due to the subjective opinions of clinicians, as explained above. The rate of CIN patients was considerably higher in this study. This study was done for the patients who have cytological abnormality on PAP smears. So, a study with a normal population may produce different results.
In conclusion, there was significant correlation between BV and the presence of CIN; however, no statistically significant relationship between BV and CIN was demonstrated by multivariate analysis. BV did not influence the severity of CIN. Moreover, there was no significant correlation between the presence of BV and HPV infection.
We performed STD screening for population control, but the number of patients positive for STD was not large enough for our statistics. However, it leaves room for further study, because other risk factors for CIN such as cigarette smoking or sexual behavior were not taken into account.
Figures and Tables
Fig. 1
Diagnosis of bacterial vaginosis (A) presence of vaginal secretions coating gray and thinly the vaginal wall, (B) vaginal pH>4.5, (C) presence of clue cells on microscopic examination of vaginal smear, (D) positive amine or whiff test.
References
1. Kim YM, Park JY, Lee KM, Kong TW, Yoo SC, Kim WY, et al. Does pretreatment HPV viral load correlate with prognosis in patients with early stage cervical carcinoma? J Gynecol Oncol. 2008. 19:113–116.
2. Uthayakumar S, Boyle DC, Barton SE, Nayagam AT, Smith JR. Bacterial vaginosis and cervical intraepithelial neoplasia: cause or coincidence? J Obstet Gynaecol. 1998. 18:572–574.
3. Boyle DC, Smith JR. Infection and cervical intraepithelial neoplasia. Int J Gynecol Cancer. 1999. 9:177–186.
4. Pavic N. Is there a local production of nitrosamines by the vaginal microflora in anaerobic vaginosis/trichomoniasis? Med Hypotheses. 1984. 15:433–436.
5. Eschenbach DA, Davick PR, Williams BL, Klebanoff SJ, Young-Smith K, Critchlow CM, et al. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol. 1989. 27:251–256.
6. Morris M, Nicoll A, Simms I, Wilson J, Catchpole M. Bacterial vaginosis: a public health review. BJOG. 2001. 108:439–450.
7. Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, et al. The Vaginal Infections and Prematurity Study Group. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N Engl J Med. 1995. 333:1737–1742.
8. Silver HM, Sperling RS, St Clair PJ, Gibbs RS. Evidence relating bacterial vaginosis to intraamniotic infection. Am J Obstet Gynecol. 1989. 161:808–812.
9. Peipert JF, Montagno AB, Cooper AS, Sung CJ. Bacterial vaginosis as a risk factor for upper genital tract infection. Am J Obstet Gynecol. 1997. 177:1184–1187.
10. Platz-Christensen JJ, Sundstrom E, Larsson PG. Bacterial vaginosis and cervical intraepithelial neoplasia. Acta Obstet Gynecol Scand. 1994. 73:586–588.
11. Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983. 74:14–22.
12. Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002. 287:2114–2119.
13. Cho NH, An HJ, Jeong JK, Kang S, Kim JW, Kim YT, et al. Genotyping of 22 human papillomavirus types by DNA chip in Korean women: comparison with cytologic diagnosis. Am J Obstet Gynecol. 2003. 188:56–62.
14. Platz-Christensen JJ, Larsson PG, Sundstrom E, Bondeson L. Detection of bacterial vaginosis in Papanicolaou smears. Am J Obstet Gynecol. 1989. 160:132–133.
15. Peters N, Van Leeuwen AM, Pieters WJ, Hollema H, Quint WG, Burger MP. Bacterial vaginosis is not important in the etiology of cervical neoplasia: a survey on women with dyskaryotic smears. Sex Transm Dis. 1995. 22:296–302.
16. Boyle DC, Barton SE, Uthayakumar S, Hay PE, Pollock JW, Steer PJ, et al. Is bacterial vaginosis associated with cervical intraepithelial neoplasia? Int J Gynecol Cancer. 2003. 13:159–163.
17. Chen KC, Amsel R, Eschenbach DA, Holmes KK. Biochemical diagnosis of vaginitis: determination of diamines in vaginal fluid. J Infect Dis. 1982. 145:337–345.
18. Frega A, Stentella P, Spera G, Pace S, Cipriano L, Di Ruzza D, et al. Cervical intraepithelial neoplasia and bacterial vaginosis: Correlation or risk factor? Eur J Gynaecol Oncol. 1997. 18:76–77.
19. Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol. 1988. 158:819–828.
20. Gravett MG, Nelson HP, DeRouen T, Critchlow C, Eschenbach DA, Holmes KK. Independent associations of bacterial vaginosis and chlamydia trachomatis infection with adverse pregnancy outcome. JAMA. 1986. 256:1899–1903.
21. Spiegel CA, Amsel R, Eschenbach D, Schoenknecht F, Holmes KK. Anaerobic bacteria in nonspecific vaginitis. N Engl J Med. 1980. 303:601–607.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download