Abstract
Gynecologic malignancies may have similar histological characteristics. This may lead to difficulties in determining the origin of the cancer and to distinguish a synchronous neoplasm from a metastatic cancer in advanced cases. Recently, we treated a 59-year-old patient with adenocarcinoma of the uterine cervix, endometrium, fallopian tubes, and ovaries. It was difficult to determine whether the cancer was a single origin metastatic cancer or a synchronous neoplasm. The patient was finally diagnosed with metastatic cancer that originated from the uterine cervix by human papillomavirus (HPV) test. Here we report the case and briefly review of the medical literature.
Metastasis of uterine cervical adenocarcinoma to the ovary is rare and uncommonly reported. In such cases, unless the primary lesion in the cervix is clear, it may be difficult to distinguish between a synchronous cancer and a metastatic cancer. Adenocarcinomas can develop from the cervix, the ovary, and the endometrium, and they may be histologically identical. However, despite similar histological characteristics, the determination of disease stage and consequently the appropriate therapeutic protocols differ depending on the tumor origin. Therefore, an accurate diagnosis must be made for appropriate treatment. Recently, we treated a patient who had lesions of the uterine cervix, the endometrium, both fallopian tubes, and the ovaries, with identical histological characteristics. It was difficult to determine the primary cancer origin from the results of surgery and histological testing. However, after detection of human papillomavirus (HPV) in the ovaries and the endometrial tissue, the patient was diagnosed with metastatic cervical adenocarcinoma.
In March 2007, a 59-year-old patient with 3-0-3-3 parity presented to our hospital with the chief complaint of blood tinged vaginal discharge for four to five months. The general condition of the patient was good and the physical examination and medical history were unremarkable. On gynecological examination, the uterus was the size of a woman's fist and irregularly shaped baseball sized masses were palpated in both adnexae. In the uterine cervix, a cauliflower shaped mass 4×4 cm in size was detected (Fig. 1). The cervical mass extended to the posterior vaginal wall and bled easily when touched. Routine blood testing, biochemical testing, urine analysis and electrocardiography were normal. The results of tumor marker analysis were as follows: carcinoembryonic antigen (CEA) 3.24 ng/ml, squamous cell carcinoma (SCC) 0.69 ng/ml, CA 19-9 16.9 u/ml, CA 125 44.70 U/ml, tissue polypeptide-specific antigen 70.03 I/U and Alpha-Fetoprotein 1.04 ng/ml. HPV testing performed on the cervix, revealed the presence of the HPV type 18. On MRI, the parametrium was not involved (Fig. 2A). The dilated endometrial cavity was filled with a poorly defined, cotton-ball like mass. Both adnexae showed multiseptated large ovarian cystic tumors suspected to be mucinous cystadenocarcinomas or borderline malignancies (Fig. 2B). Such findings made it difficult to determine the primary organ from which the cancer developed. We performed gastroendoscopy and colonoscopy and both were also negative. Considering the possibility of both synchronous cancer and metastatic cancer, type III radical hysterectomy with bilateral salpingo-oophorectomy, pelvic lymph node dissection and omentectomy were performed.
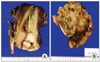
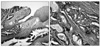
By macroscopic examination, the masses from the uterine cervix and the endometrium revealed a thickened papillary growth (Fig. 3A). Both ovaries were multicystic masses with cavities filled with mucin. Both fallopian tubes were hardened and expanded to 1.3 cm in diameter; a light brown papillary structure protruded toward the inner cavity during resection (Fig. 3B). Microscopically, the tumor is composed of malignant glands showing papillary, micropapillary and cribriform growth (Fig. 4). The ovarian capsule was intact other than the local infiltration. Among 25 pelvic lymph nodes, metastases were detected in 11 lymph nodes. The immunohistochemical staining performed on the uterine cervix and the ovarian mass had an identical staining pattern that was vimentin negative, CEA negative and CK7 negative. CK20 was positive in only some areas of the uterine cervix. The possibility of a primary ovarian cancer was thought to be relatively low, because despite its advanced stage there was no accompanying carcinomatous peritonei and the infiltration into the ovarian capsule was local. The possibility of uterine endometrial cancer was also assessed to be low because the endometrial lesion showed diffuse infiltration without forming a distinct mass, and the immunohistochemical findings with CEA and vimentin. Metastases from a colorectal carcinoma are usually CK20 positive, but we could not find any lesion in the colon or the rectum during operation. Nevertheless, the possibility of an ovarian cancer or metastatic cancer from remote organs could not be completely ruled out, and we needed further study to confirm the diagnosis. Finally, we evaluated the HPV status using the HPV DNA chip and demonstrated that all lesions in the uterus and the ovary were HPV 18 positive. Therefore, the patient was diagnosed with stage IV cervical adenocarcinoma with metastases. The patient is receiving combination chemotherapy with platinum and taxane.
Even if the primary origins are different, cancer from the female reproductive organs may show similar histological characteristics. Cervical adenocarcinomas do not commonly metastasize to the ovaries. They usually exhibit endometrioid and/or mucinous differentiation. Due to their morphologic similarity to the common mucinous and endometrioid subtypes of primary ovarian cancer or even endometrial cancer, it may be difficult to distinguish a synchronous neoplasm from an ovarian metastasis simulating a primary ovarian neoplasm. Even minimally invasive endocervical adenocarcinomas may metastasize to the ovary and other adjacent organs, making the diagnosis of the tumor origin more difficult.1
However, ovarian metastases from cervical adenocarcinomas are rare; most cases of synchronous ovarian and cervical adenocarcinomas reported have been determined to be independent primary cervical and ovarian neoplasms. Because the disease stage and consequent treatment protocols differ depending on the primary origin of the cancer, inaccurate assessment of the primary lesion may hamper appropriate patient treatment. Therefore, there are many trials ongoing for accurate diagnosis. One of the most widely used diagnostic tools is the immunohistochemical method to detect antigens specific to a distinct cancer. However, in advanced stage cancer, such immunohistochemical characteristics become less definitive.2
The role of HPV in the development of uterine cervical squamous cell carcinoma has been well characterized. According to recent studies, adenocarcinomas that account for 20-25% of cervical cancers have been shown to be associated with HPV in more than 90% of cases. HPV is thought to have an etiologic role similar to the role of HPV in squamous cell carcinoma.3 In cases of cervical adenocarcinoma, attention has been focused on the association of HPV 18. Investigators have recently reported that HPV 18 has a specific diagnostic value for cervical adenocarcinoma.4 In contrast to cervical cancer, the relation of HPV and other gynecological cancers such as ovarian or endometrial cancer has not been accepted to date. Some studies have reported that HPV has been isolated from ovarian cancer tissues, although at a lower rate than cervical cancer.5,6 But in most studies, HPV was not isolated, or isolated only in some cases of ovarian cancer, and the likelihood that HPV plays a role in ovarian carcinogenesis is thought to be low.4,7,8 This means that identification of HPV DNA in ovarian tumors may provide evidence of a metastatic cancer.2,9,10 Reports of cases with an occult cervical adenocarcinoma identified after the detection of HPV in ovarian tissues support the utility of HPV for diagnosis.9 In endometrial cancer it has been reported that the rate of the isolation of HPV was higher than in the ovary. But similar to ovarian cancer, a direct association to HPV has not been confirmed.2,7
In our case, identical HPV 18 expression was identified in cancer tissue from the ovary and the endometrium, and as a result, metastatic cervical cancer was diagnosed. HPV testing along with clinical characteristics may help identify the primary tumor origin in similar cases.
Figures and Tables
Fig. 1
Colposcopic appearance of the cervical mass shows surface irregularity and atypical blood vessels prone to bleeding.
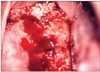
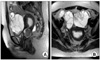
Fig. 2
Sagittal T2-weighted image (A) shows a lobulated mass in the uterine cervix with heterogeneous signal intensity. On the axial T2-weighted image (B), multiseptated cystic masses with multiple irregular septa and some solid portions were detected in both adnexa. A dilated endometrial cavity with poorly defined, cotton-ball like endometrial surface represents endometrial spreading of the tumors.
References
1. Elishaev E, Gilks CB, Miller D, Srodon M, Kurman RJ, Ronnett BM. Synchronous and metachronous endocervical and ovarian neoplasms: evidence supporting interpretation of the ovarian neoplasms as metastatic endocervical adenocarcinomas simulating primary ovarian surface epithelial neoplasms. Am J Surg Pathol. 2005. 29:281–294.
2. Park TW, Zivanovic O, Theuerkauf I, Durkop B, Hernando JJ, Simon M, et al. The diagnostic utility of human papillomavirus-testing in combination with immunohistochemistry in advanced gynaecologic pelvic tumours: a new diagnostic approach. Int J Oncol. 2004. 24:829–836.
3. Andersson S, Rylander E, Larsson B, Strand A, Silfversvärd C, Wilander E. The role of human papillomavirus in cervical adenocarcinoma carcinogenesis. Eur J Cancer. 2001. 37:246–250.
4. Yang HJ, Liu VW, Tsang PC, Yip AM, Ng TY, Cheung AN, et al. Comparison of human papillomavirus DNA levels in gynecological cancers: implication for cancer development. Tumour Biol. 2003. 24:310–316.
5. Wu QJ, Guo M, Lu ZM, Li T, Qiao HZ, Ke Y. Detection of human papillomavirus-16 in ovarian malignancy. Br J Cancer. 2003. 89:672–675.
6. Ip SM, Wong LC, Xu CM, Cheung AN, Tsang PC, Ngan HY. Detection of human papillomavirus DNA in malignant lesions from Chinese women with carcinomas of the upper genital tract. Gynecol Oncol. 2002. 87:104–111.
7. Hisada M, van den Berg BJ, Strickler HD, Christianson RE, Wright WE, Waters DJ, et al. Prospective study of antibody to human papilloma virus type 16 and risk of cervical, endometrial, and ovarian cancers (United States). Cancer Causes Control. 2001. 12:335–341.
8. Anttila M, Syrjänen S, Ji H, Saarikoski S, Syrjanen K. Failure to demonstrate human papillomavirus DNA in epithelial ovarian cancer by general primer PCR. Gynecol Oncol. 1999. 72:337–341.
9. Powell JL, Bock KA, Gentry JK, White WC, Ronnett BM. Metastatic endocervical adenocarcinoma presenting as a virilizing ovarian mass during pregnancy. Obstet Gynecol. 2002. 100(5 Pt 2):1129–1133.
10. Plaza JA, Ramirez NC, Nuovo GJ. Utility of HPV analysis for evaluation of possible metastatic disease in women with cervical cancer. Int J Gynecol Pathol. 2004. 23:7–12.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download