Abstract
Objective
To determine the factors associated with tumor volume response to radiotherapy (RT) in cervical cancer patients, and the relationship between the tumor volume response and alteration of the expression of biological markers during RT.
Methods
Twenty consecutive patients with cervical squamous cell carcinoma who received definitive RT were enrolled. Tumor volumes were calculated by MRI examinations performed at the start of RT (pre-RT), at the fourth week of RT (mid-RT), and 1 month after RT completion (post-RT). Two serial punch biopsies were performed at pre- and mid-RT, and immunohistochemical staining was performed for cyclooxygenase (COX)-2 and epidermal growth factor receptor (EGFR).
Results
For the pre-RT evaluation, fourteen (70%) and eleven (55%) patients showed positive immunoreactivity for COX-2 and EGFR, respectively. Among the seven patients whose median percentage residual tumor at mid-RT (V2R) was greater than 0.5, seven (100%, p=0.0515) and five (71.4%, p=0.3742) patients showed positive immunoreactivity for COX-2 and EGFR, respectively. The logistic regression analysis showed that positive immunoreactivity for both COX-2 and EGFR at pre-RT were associated with V2R (p=0.0782). For the mid-RT evaluation, eight cases showed an interval increase in the distribution of immunoreactivity for COX-2, and six out of the eight patients had a V2R greater than 0.5 (p=0.2222).
Uterine cervical cancer is the second most common female malignancy in the world. It occurs almost two-fold higher in less developed countries compared with more-developed countries.1 For patients with uterine cervical cancer, radiation therapy (RT) is an important treatment modality in a variety of clinical situations. Especially in early cervical cancer patients, surgery and RT are equivalent in terms of clinical outcome, and the treatment choice depends on the patient's age, co-morbidities, etc. On the other hand, in locally advanced cervical cancer patients, concurrent chemoradiotherapy represents the standard treatment, RT alone being reserved for special conditions.2-5
In addition to FIGO stage, pelvic or para-aortic lymph node status, initial tumor size, and post-RT tumor size are well-established prognostic factors and predictors of outcome after RT in patients with cervical cancer.6-8 Some investigators have suggested that the tumor volume response, assessed at 4-5 weeks after initiation of RT, is associated with local disease control and disease free survival.9,10 Recently, several studies have reported that specific biological markers, such as cyclooxygenase (COX)-2, epidermal growth factor receptor (EGFR), and vascular endothelial growth factor (VEGF) are significantly correlated with tumor control, survival after RT with or without chemotherapy.11-18
The goals of this prospective study were 1) to determine the factors associated with the tumor volume response to RT with or without chemotherapy and 2) to determine the relationship between the tumor volume response and alteration of the expression of the biological markers during RT with or without chemotherapy.
Twenty consecutive patients with squamous cell carcinoma of the uterine cervix who received definitive RT, with or without chemotherapy, at Samsung Medical Center between March 2005 and November 2006 were evaluated (Table 1). When pelvic lymph nodes exceeded 1.5 cm in diameter and/or there was significant F18-fluorodeoxyglucose uptake by positron emission tomography (PET), the lymph node was considered positive for cancer involvement. Pre-treatment PET was performed in 12 patients. Among them, seven patients had significant abnormal uptake in pelvic lymph nodes. The institutional review board approved this study and all patients voluntarily participated in this study by signing the informed consent.
All patients received a combination of external beam radiotherapy (EBRT) and high-dose-rate (HDR) intracavitary irradiation (ICR) by a remote after loading system with Ir-192. EBRT was delivered to the whole pelvis with 15-MV photon beams at a daily dose of 1.8 Gy, five times per week to a total dose of 50.4 Gy. The patients were irradiated with a 4-field box technique in order to spare the small bowel anterior to the iliac nodes. Extended field irradiation including the para-aortic area, with a total dose of 45 Gy, was adminstered for one patient with positive common iliac lymph nodes. HDR ICR was initiated after an EBRT dose of 41.4 Gy to 45 Gy with midline blocking. ICR was delivered twice a week in six fractions with a fractional dose of 4 Gy at point A. The median overall treatment time was 53 days (range, 46 to 61 days). Three patients with FIGO stage IB or IIA disease who had small tumors less than 3 cm did not receive concurrent chemotherapy. The remaining seventeen patients received concurrent chemoradiotherapy. Eleven patients received six cycles of weekly cisplatin (30 mg/m2) during RT. The remaining six patients were treated with a 5-fluorouracil (1,000 mg/m2) plus cisplatin (60 mg/m2) regimen every 3-4 weeks for 2 or 3 cycles during RT, and additional chemotherapy were administered after RT for 1 or 3 cycles. The selection of the chemotherapeutic regimen was individualized according to local tumor extent, pelvic lymph node involvement, and patient's general condition. During treatment, assessment of acute toxicity was performed every week using the Radiation Therapy Oncology Group (RTOG) criteria.
Serial MRI examinations were performed at three time points: at the start of the RT (pre-RT), at the fourth week of RT (mid-RT), and 1 month after completion of RT (post-RT). MRI was performed with a 1.5-T MR system (Signa; GE Medical Systems, Milwaukee, WI, USA) using a phased array or body coil. The pelvis was imaged with an axial fast spinecho T2-weighted sequence with an echo train length of 20 to 25, a 5-mm-section thickness, and a 2-mm intersection gap. The matrix size was 512×256 and the field of view was 28 to 30 cm. Three-dimensional tumor volumes were calculated by a radiation oncologist without knowledge of the FIGO stage and tumor marker status. On T2-weighted images, the area of the tumor in each slice was outlined and the tumor volume was calculated by summation of all the areas of tumor and multiplied by the slice interval. Tumor volume responses were calculated by dividing the tumor volume at a given point by the pre-RT tumor volume (V1),
where V2 was the mid-RT tumor volume, V3 was the post-RT tumor volume; V2R and V3R were the percentage of residual tumor volume at each time.
Serial punch biopsies were performed at pre-RT and mid-RT with intent to obtain tumor tissues adequately. After verifying the presence of the tumor on the hematoxylin and eosin stained slides, immunohistochemical staining was performed for COX-2 and EGFR. Formalin-fixed, paraffin-embedded 5 µm-thick sections were prepared from the biopsy specimens. The slides were deparaffinized in xylene and endogenous peroxidase was blocked by incubating the slides in 0.3% H2O2 for 15 minutes. Antigen retrieval was performed using 0.01 M citrate buffer for COX-2 and proteinase K for EGFR, respectively. Incubation of the sections in a solution of 4% bovine serum and phosphate buffered saline dilution for 30 minutes was performed to block nonspecific binding. Then, the specimen was incubated with the primary antibody. The COX-2 antibody was purchased from Cayman (Ann Arbor, MI, USA) and the EGFR antibody from Dako (Carpinteria, CA, USA). The sections were incubated with anti-mouse secondary antibodies of the ChemaMate TM detection kit from Dako. Deaminobenzidine was used as the chromogen, and hematoxylin was used as the nuclear counter stain.
Two experienced pathologists, without knowledge of the clinical information, examined the sections under the light microscope and evaluated the distribution of the immunoreactivity. The intensity of staining was classified as negative indicating no expression, weak indicating minimal staining, moderate indicating moderate staining, and strong representing heavy staining. If the distribution of moderate to strong immunoreactivity accounted for more than 10%, the specimen was classified as positive. If there was an available mid-RT immunohistochemical stained slide, the distribution of moderate to strong immunoreactivity was compared with that of the pre-RT specimen. The alteration of the distribution of immunoreactivity was analyzed.
The relationship between the distribution of immunoreactivity for COX-2 and EGFR was assessed by Pearson's correlation. Pearson's correlation was also applied for assessing the relationship between V1 and V2R. Correlations between tumor volume parameters (V1, V2R, V3R) and biological markers (COX-2, EGFR) were examined by Fisher's exact test. For identifying factors associated with the mid-RT tumor volume response (V2R), a multiple logistic regression analysis was employed including V1, FIGO stage, and positivity for both biological markers. The SAS ver. 9.0 (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis. A p-value ≤ 0.05 was considered statistically significant.
The median tumor volume at pre-RT was 20.9 cm3 (range, 1.7 to 132.3 cm3), which regressed to 9.3 cm3 (range, 0.4 to 73.5 cm3) at mid-RT. The percentage of residual tumor volume at mid-RT (V2R) ranged from 0.136 to 0.983 (median 0.396). The relationship between V1 and V2R was not statistically significant (Pearson correlation coefficient=.0.1376, p=0.5629) (Fig. 1). At post-RT, 15 (75%) patients showed complete regression of tumor on MRI. The remaining five (25%) patients had residual tumor that ranged from 1.7 cm3 to 6.6 cm3.
For the pre-RT evaluation, fourteen (70%) and eleven (55%) patients showed positive immunoreactivity for COX-2 and EGFR, respectively (Fig. 2). Among them, eight patients (40%) showed positive immunoreactivity for both biological markers. The relationship between the distribution of immunoreactivity for the biological markers was not statistically significant (Pearson correlation coefficient=0.2985, p=0.2011).
For the mid-RT evaluation, there were only twelve available immunohistochemical stained slides for COX-2 and EGFR (60%). The reasons that not all patients had slides available for review included tumor necrosis by radiation and/or sampling errors. Among the twelve available slides at mid-RT, eight (67%) cases for COX-2 and six (50%) cases for EGFR showed an interval increase in the distribution of immunoreactivity, compared with immunoreactivity of the pre-RT evaluation.
Among the seven patients whose V2R was greater than 0.5, all seven (100%) showed positive immunoreactivity for COX-2, and five (71.4%) for EGFR at the pre-RT evaluation (Table 2). The relationship between the V2R and the pre-RT COX-2 expression was marginally significant (p=0.0515). There was no significant relationship between the other volume factors (V1, V3R) and the biological markers. Among the five patients whose V3R was greater than 0, three (60%) and two (40%) showed positive immunoreactivity for COX-2 and EGFR, respectively.
Among the eight patients who showed positive immunoreactivity for both biological markers, a V2R greater than 0.5 was observed in five patients (63%) compared with the rest (17%). However, the difference was only marginally significant (p=0.0623). The logistic regression analysis showed that positive immunoreactivity for both biological markers was associated with the V2R (p=0.0782), which was also marginally significant (Table 3).
Among the eight patients who showed an interval increase in the distribution of immunoreactivity for COX-2 at mid-RT compared with immunoreactivity for COX-2 at pre-RT, six (75%) patients had V2R greater than 0.5 compared with the patients who showed no change or a decrease in the distribution of COX-2 expression (25%) (Table 4). However, the finding was not statistically significant (p=0.2222). Two out of the six patients showed residual tumor at the post-RT evaluation. There were no significant findings for the interval change in the distribution of immunoreactivity for EGFR (data not shown).
There was no incidence of RTOG grade 3 or higher radiation related toxicity. Six patients experienced grade 2 toxicity, five experienced lower gastrointestinal and one genitourinary toxicity. Among the six patients, four patients showed positive immunoreactivity for COX-2 at the pre-RT evaluation and the remaining two patients did not.
COX-2 is a key enzyme that catalyzes the conversion of pro-staglandins and other eicosanoids from arachidonic acid. COX-2 is rapidly inducible in response to numerous intracellular and extracellular stimuli, and acts in a pro-inflammatory fashion. It promotes carcinogenesis, tumor pro-liferation and growth.19-21 Several reports have shown that COX-2 is associated with adverse outcome after treatment of cervical cancer.11,12,18,22 Gaffney et al.11 reported that increased expression of COX-2 was associated with decreased overall survival (p=0.021) and disease free survival (p=0.015). Kim et al.12 also showed a decreased overall survival (p= 0.003) and disease free survival in their COX-2 positive group.
The results of this study suggest that expression of COX-2 negatively affected the tumor volume response during RT, a known adverse prognostic factor for cervical cancer.9,10 The findings of increased distribution of immunoreactivity during RT support the conclusion that COX-2 expression had a negative influence on the volume response to radiotherapy. Also, Ferrandina et al.14 showed a strong correlation between COX-2 expression and clinical tumor response to neoadjuvant chemotherapy in patients with cervical cancer.
EGFR has been shown to play a role in cell differentiation, enhancement of cell motility, protein secretion, neovascularization, invasion, metastasis, and resistance of cancer cells to chemotherapeutic agents and radiation.23-25 The negative association of EGFR with cervical cancer patient prognosis has been previously reported.13,15,17 Kim et al.13 reported that overexpression of EGFR was associated with a decreased overall survival (p=0.04) and disease free survival (p=0.03).
However, the results of this study did not demonstrate a significant relationship between EGFR expression and tumor volume response. In addition, there was no significant relationship between the distribution of immunoreactivity for EGFR and COX-2 expression (p=0.2011).
In this study, coexpression of COX-2 and EGFR showed marginally significant relationship with poor mid-RT volume response. Kim et al.16 reported a similar finding regarding the coexpression of these two markers. The synchronous coexpression of EGFR and COX-2 was a more significant and independent prognostic factor for predicting poor survival in FIGO stage IIB cervical cancer. Several studies have revealed a relationship between COX-2 and EGFR.20,26,27 Activation of the EGFR signaling pathway enhances transforming growth factor expression, which results in increased transcription of COX-2. P38 mitogen-activated protein kinase is the major signaling pathway that contributes to EGFR-dependent COX-2 induction.26,27 Given these findings, these two markers may be targets for novel therapies. The combination of Celecoxib®, a COX-2 selective inhibitor, and Gefitinib®, an EGFR tyrosine kinase (TK) inhibitor, has shown synergistic inhibition of cell growth in squamous cell carcinoma of the head and neck and lung cancer cells.28,29 A recent prospective trial of celecoxib alone in combination with definitive chemoradiotherapy (CRT), in women with locally advanced cervical cancer, was unable to demonstrate better efficacy than CRT alone,30 the combination of a COX-2 inhibitor and an EGFR TK inhibitor might provide a new therapeutic option for patients with cervical cancer.
Although the number of patients in this study was small, the poor mid-RT tumor volume response may be associated with COX-2 expression and with coexpression of COX-2 and EGFR. Furthermore, there was an increase in the intrinsic radioresistance associated with COX-2 expression. The treatment administered can be altered based on these findings. To overcome the adverse effects of a poor volume response, individualized radiation dose escalation or consolidation chemotherapy may be alternatives to conventional CRT. In addition, targeted therapy according to the patient's status of biological marker expression may provide additional treatment options.
Figures and Tables
Fig. 1
The relationship between the pre-radiotherapy tumor volume and the percentage residual tumor volume at mid-radiotherapy.
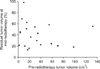
Fig. 2
Representative slides with immunoreactivity for biological markers. Panel (A) (×400) is a slide with positive expression of cyclooxygenase (COX)-2, and panel (B) (×400) with negative expression of COX-2. Positive and negative expression of epidermal growth factor receptor (EGFR) is shown in Panel (C) and (D) (×200), respectively.
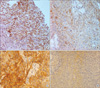
Table 3
Multiple logistic regression analysis for percentage of residual tumor volume at mid-radiotherapy (V2R)

References
1. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006. 24:2137–2150.
2. Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997. 350:535–540.
3. Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL 3rd, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999. 340:1154–1161.
4. Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999. 340:1137–1143.
5. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999. 340:1144–1153.
6. Lowrey GC, Mendenhall WM, Million RR. Stage IB or IIA-B carcinoma of the intact uterine cervix treated with irradiation: a multivariate analysis. Int J Radiat Oncol Biol Phys. 1992. 24:205–210.
7. Eifel PJ, Morris M, Wharton JT, Oswald MJ. The influence of tumor size and morphology on the outcome of patients with FIGO stage IB squamous cell carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1994. 29:9–16.
8. Kapp KS, Stuecklschweiger GF, Kapp DS, Poschauko J, Pickel H, Lahousen M, et al. Prognostic factors in patients with carcinoma of the uterine cervix treated with external beam irradiation and IR-192 high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 1998. 42:531–540.
9. Mayr NA, Taoka T, Yuh WT, Denning LM, Zhen WK, Paulino AC, et al. Method and timing of tumor volume measurement for outcome prediction in cervical cancer using magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2002. 52:14–22.
10. Nam H, Park W, Huh SJ, Bae DS, Kim BG, Lee JH, et al. The prognostic significance of tumor volume regression during radiotherapy and concurrent chemoradiotherapy for cervical cancer using MRI. Gynecol Oncol. 2007. 107:320–325.
11. Gaffney DK, Holden J, Davis M, Zempolich K, Murphy KJ, Dodson M. Elevated cyclooxygenase-2 expression correlates with diminished survival in carcinoma of the cervix treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2001. 49:1213–1217.
12. Kim YB, Kim GE, Cho NH, Pyo HR, Shim SJ, Chang SK, et al. Overexpression of cyclooxygenase-2 is associated with a poor prognosis in patients with squamous cell carcinoma of the uterine cervix treated with radiation and concurrent chemotherapy. Cancer. 2002. 95:531–539.
13. Kim YT, Park SW, Kim JW. Correlation between expression of EGFR and the prognosis of patients with cervical carcinoma. Gynecol Oncol. 2002. 87:84–89.
14. Ferrandina G, Ranelletti FO, Legge F, Lauriola L, Poerio A, Zannoni GF, et al. Cyclooxygenase-2 (COX-2) expression in locally advanced cervical cancer patients undergoing chemoradiation plus surgery. Int J Radiat Oncol Biol Phys. 2003. 55:21–27.
15. Gaffney DK, Haslam D, Tsodikov A, Hammond E, Seaman J, Holden J, et al. Epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) negatively affect overall survival in carcinoma of the cervix treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2003. 56:922–928.
16. Kim GE, Kim YB, Cho NH, Chung HC, Pyo HR, Lee JD, et al. Synchronous coexpression of epidermal growth factor receptor and cyclooxygenase-2 in carcinomas of the uterine cervix: a potential predictor of poor survival. Clin Cancer Res. 2004. 10:1366–1374.
17. Lee CM, Shrieve DC, Zempolich KA, Lee RJ, Hammond E, Handrahan DL, et al. Correlation between human epidermal growth factor receptor family (EGFR, HER2, HER3, HER4), phosphorylated Akt (P-Akt), and clinical outcomes after radiation therapy in carcinoma of the cervix. Gynecol Oncol. 2005. 99:415–421.
18. Ishikawa H, Ohno T, Kato S, Wakatsuki M, Iwakawa M, Ohta T, et al. Cyclooxygenase-2 impairs treatment effects of radiotherapy for cervical cancer by inhibition of radiation-induced apoptosis. Int J Radiat Oncol Biol Phys. 2006. 66:1347–1355.
19. Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999. 18:7908–7916.
20. Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000. 69:145–182.
21. Williams CS, Tsujii M, Reese J, Dey SK, DuBois RN. Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest. 2000. 105:1589–1594.
22. Ferrandina G, Lauriola L, Distefano MG, Zannoni GF, Gessi M, Legge F, et al. Increased cyclooxygenase-2 expression is associated with chemotherapy resistance and poor survival in cervical cancer patients. J Clin Oncol. 2002. 20:973–981.
23. Huang SM, Harari PM. Epidermal growth factor receptor inhibition in cancer therapy: biology, rationale and preliminary clinical results. Invest New Drugs. 1999. 17:259–269.
24. Wells A. EGF receptor. Int J Biochem Cell Biol. 1999. 31:637–643.
25. Oh MJ, Choi JH, Kim IH, Lee YH, Huh JY, Park YK, et al. Detection of epidermal growth factor receptor in the serum of patients with cervical carcinoma. Clin Cancer Res. 2000. 6:4760–4763.
26. Matsuura H, Sakaue M, Subbaramaiah K, Kamitani H, Eling TE, Dannenberg AJ, et al. Regulation of cyclooxygenase-2 by interferon gamma and transforming growth factor alpha in normal human epidermal keratinocytes and squamous carcinoma cells: role of mitogen-activated protein kinases. J Biol Chem. 1999. 274:29138–29148.
27. Xu K, Shu HK. EGFR activation results in enhanced cyclooxygenase-2 expression through p38 mitogen-activated protein kinase-dependent activation of the Sp1/Sp3 transcription factors in human gliomas. Cancer Res. 2007. 67:6121–6129.
28. Chen Z, Zhang X, Li M, Wang Z, Wieand HS, Grandis JR, et al. Simultaneously targeting epidermal growth factor receptor tyrosine kinase and cyclooxygenase-2, an efficient approach to inhibition of squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004. 10:5930–5939.
29. Park JS, Jun HJ, Cho MJ, Cho KH, Lee JS, Zo JI, et al. Radiosensitivity enhancement by combined treatment of celecoxib and gefitinib on human lung cancer cells. Clin Cancer Res. 2006. 12:4989–4999.
30. Herrera FG, Chan P, Doll C, Milosevic M, Oza A, Syed A, et al. A prospective phase I-II trial of the cyclooxygenase-2 inhibitor celecoxib in patients with carcinoma of the cervix with biomarker assessment of the tumor microenvironment. Int J Radiat Oncol Biol Phys. 2007. 67:97–103.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download