Abstract
Primary ovarian choriocarcinoma arising from a germ cell is an extremely rare occurrence, especially in postmenopausal women, and the prognosis is poor. Non-gestational choriocarcinoma of the ovary (NGCO) accounts for 0.6% or less of all ovarian neoplasms. It is important to distinguish gestational choriocarcinomas of the ovary (GCO) from other carcinomas because of the poor prognosis of NGCO. We describe a case of NGCO with lung metastasis in a 55 year old woman, which we present together with a brief review of the literature.
Most cases of choriocarcinoma occur in the uterine body and stem from chorionic villi following a normal or abnormal gestation. Most instances of choriocarcinoma of the ovary are gestational in origin. In contrast, non-gestational choriocarcinoma of the ovary (NGCO) is an exceedingly rare primary germ cell neoplasm that has a worse prognosis than gestational neoplasms, and most patients show metastasis to organ parenchyma at the time of diagnosis. NGCO accounts for 0.6% or less of all ovarian neoplasms.1 It is important to distinguish between gestational choriocarcinoma of the ovary (GCO) and other carcinomas because of the poor prognosis of NGCO. No distinctive ultrastructural or immunohistochemical differences have been found between NGCO and GCO. Cytogenetic studies may be appropriate for the investigation of the potential reasons for the difference in prognosis.
We describe a case of NGCO with lung metastasis which originated in the ovary of a postmenopausal woman together with a brief review of the literature.
A-55-year-old woman (gravida 5, para 3) with intermittent pelvic pain and a dry cough was referred to the Department of obstetrics and gynecology at the Daegu Catholic Medical Center. She complained of intermittent pelvic pain and a dry cough for 1 year. Recently her symptoms changed for the worse, which forced her to visit a local medical center. She had no specific family history of medical disease. Her last menstrual period was 5 years ago. Her husband died 10 years ago and she has had no sexual contact since then.
At the time of admission, the general condition of the patient appeared to be relatively good; blood pressure was 127/84 mmHg, the pulse rate was 82/min and the body temperature was 36.5℃. Speculum examination revealed a closed cervix. The uterus was anteverted and small. A mass of approximately the size of a goose egg in the right adnexal region was palpated. Transvaginal ultrasound demonstrated a mass measuring 61×48 mm in the right adnexal region. Laboratory tests showed no specific findings. Tumor markers, namely CA125, β-human chorionic gonadotropin (β-hCG), and α-feto protein (AFP), were 26 U/ml, 64,838 mIU/ml, and 3.3 ng/ml, respectively. Computed tomography (CT) and x-ray of the chest showed multiple round shaped nodules in the whole lung field. During laparotomy, a mass was located in the right adnexal region. Other peritoneal surfaces, including the uterus, both of the ovaries, and the fallopian tubes were macroscopically normal. Total abdominal hysterectomy, bilateral salpingo-oophorectomy and multiple biopsies were performed.
On macroscopic examination of the mass specimen, a dark red fleshy, hemorrhagic and necrotic mass with a smooth surface, measuring 8×5×4 cm in diameter, was visible. The mass was attached to the right fallopian tube. Histopathologic examination showed that the tumor was composed of clusters and sheets of neoplastic mononuclear cytotrophoblastic and multinucleated syncytiotrophoblastic cells in an extensively necrotic background (Fig. 1). Immunohistochemically, strong cytoplastic immunoactivity for hCG was found in the syncytiotrophoblastic cells. There were no specific findings except for senile changes in the uterine cavity and in both ovaries.
After the operation, three courses of chemotherapy, including protocol bleomycin 25 mg, etoposide 100 mg/m2, and cisplatin 20 mg/m2 were initiated. The β-hCG level returned to normal after cycle 3 and remained normal thereafter. A chest X-ray showed decreased number of nodules. At eight months after chemotherapy, the patient was disease free. At 6 months after the completion of treatment, the PET scan produced no abnormal findings. Until now, the patient, who is still under follow up, has shown no recurrence 20 months after the completion of treatment.
Choriocarcinoma is categorized as either gestational or nongestational. Gestational choriocarcinoma can occur following a pregnancy and is characterized by its presentation in the uterine corpus. On the other hand, nongestational choriocarcinoma is thought to arise from germ cells and is characterized by its presentation in males and children. The distinction between NGCO and GCO is warranted because of the worse prognosis of NGCO. NGCO requires more aggressive therapy with multiple chemotherapeutic agents.1
It is difficult to diagnose ovarian choriocarcinoma as gestational or nongestational except in patients who are sexually immature, unable to conceive, or who have never had sexual intercourse. DNA analysis is a reliable method for distinguishing gestational tumors from nongestational tumors. Tsujioka et al.2 reported that the genetic origin could be determined by using only two or three appropriate variable numbers of tandem repeat loci. Our patient had no sexual contact since her husband's death, and therefore we thought that her case may be a NGCO. And she had no connection between her child, so we could not obtain informed consent from the patient.
A comparison of the number of case reports of ovarian choriocarcinoma is presented in Table 1.3-10 The electron microscopic characteristics of gestational choriocarcinoma have been well documented. The current case displayed no significant ultrastructural differences with respect to gestational choriocarcinoma.
Ovarian choriocarcinoma, an exceeding rare germ cell tumor, is considered to be aggressive in nature and most patients with this tumor are usually young.11
Choriocarcinoma can arise in one of three ways: as a primary choriocarcinoma associated with ovarian pregnancy; as a metastatic choriocarcinoma from a primary gestational choriocarcinoma arising in other parts of the genital tract, mainly the uterus; and as a germ cell tumor differentiating in the direction of trophoblastic structures and admixed with other neoplastic germ cell elements. A tumor arising in the latter way is classified as a non-gestational choriocarcinoma.
As these tumors are rare, treatment recommendations for primary extraovarian, nongestational choriocarcinoma are not available. Weiss et al.11 emphasized that there were two options for the treatment of these tumors. One of them was to treat this type of tumor as a gestational germ cell tumor and then decide whether to initiate single-agent or multi agent chemotherapy. The second option was to treat the patient with a germ cell tumor protocol, such a VAC (vincristine/actinomycin D/cyclophosphamide) or BEP (bleomycin/etoposide/ cisplatinum).
We initiated the treatment with multi agent chemotherapy, and the response was positive. The serum β-hCG level fell to within the normal range. All choriocarcinomas secrete hCG, which can be useful for monitoring the patient's response to treatment. However, the serum β-hCG level did not allow us to distinguish between gestational and nongestational disease. The presence of paternal DNA in the tumor can be used to distinguish gestational from non-gestational choriocarcinomas. However, it is limited to clinical applications. Serum β-hCG should be determined weekly during treatment, then every 2 weeks for 3 months, then monthly for next 3 months, and then every 2 months for the next 6 months. If the treatment fails to elicit a continued fall in β-hCG titers, or if disease recurs after cessation of chemotherapy, then the patient should be reevaluated, and therapy different from that previously employed should be initiated. Alternatively, if the β-hCG level reaches an undetectable level, treatment should be continued for two more courses in the case of gestational disease, but more extended treatment should be considered in the case of a nongestational disease.12
These tumors usually behave more like germ cell choriocarcinomas with rapid and extensive hematogenous dissemination. Thus, they spread widely to other organs and have a poor outcome.13 However, in our case, as the response to multiple agent chemotherapy was positive, the prognosis was good.
In this report, a case of nongestational choriocarcinoma with a pelvic mass and multiple lung metastasis was treated by surgery in combination with three courses of chemotherapy.
Figures and Tables
Fig. 1
Microscopic findings: the tumor is composed of syncytiotrophoblast and cytotrophoblast with hemorrhage (H-E stain, ×100).
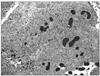
Table 1
Ovarian choriocarcinoma: summary of cases

β-hCG: β-human chorionic gonadotropin, CTx: chemotherapy, TAH: total abdominal hysterectomy, BSO: both salpingooophorectomy, EMA CO: etoposide, methotrexate, leucovorin, dactinomycin, cyclophosphamide, vincristine, NED: no evidence of disease, LO: left oophorectomy, MTX: methotrexate, VBP: vinblastine, bleomycin, cisplatin, RO: right oophorectomy, BEP: bleomycin, etoposide, cisplatin, RSO: right salpingooophorectomy, MAC: methotrexate, actinomycin-D, cyclophosphamide.
References
1. Jacobs AJ, Newland JR, Green RK. Pure choriocarcinoma of the ovary. Obstet Gynecol Surv. 1982. 37:603–609.
2. Tsujioka H, Hamada H, Miyakawa T, Hachisuga T, Kawarabayashi T. A pure nongestational choriocarcinoma of the ovary diagnosed with DNA polymorphism analysis. Gynecol Oncol. 2003. 89:540–542.
3. Yamamoto E, Ino K, Yamamoto T, Sumigama S, Nawa A, Nomura S, et al. A pure nongestational choriocarcinoma of the ovary diagnosed with short tandem repeat analysis: case report and review of the literature. Int J Gynecol Cancer. 2007. 17:254–258.
4. Jacobs AJ, Newland JR, Green RK. Pure choriocarcinoma of the ovary. Obstet Gynecol Surv. 1982. 37:603–609.
5. Vance RP, Geisinger KR. Pure nongestational choriocarcinoma of the ovary: report of a case. Cancer. 1985. 56:2321–2325.
6. Byeun TS, Byeun C, Chung DY, Park TC, Ahn WS, Lee JW, et al. A case of primary ovarian carcinoma. Korean J Obstet Gynecol. 1995. 38:1713–1717.
7. Kim KS, Kim JH, Jung MJ, Oh BC. Primary ovarian non-gestational choriocarcinoma in a young woman. Korean J Obstet Gynecol. 1997. 40:1802–1807.
8. Shin YS, You HI, Lim OR, Park SY, Kim YT, Lee KW. A case of primary ovarian choriocarcinoma. Korean J Obstet Gynecol. 1994. 37:592–596.
9. Kim PS, Kim SC, Kim JH, Choi YM, Lee HP. Pure choriocarcinoma of ovary. Korean J Obstet Gynecol. 1990. 33:1607–1611.
10. Hirabayashi K, Yasuda M, Osamura RY, Hirasawa T, Murakami M. Ovarian nongestational choriocarcinoma mixed with various epithelial malignancies in association with endometriosis. Gynecol Oncol. 2006. 102:111–117.
11. Weiss S, Amit A, Schwartz MR, Kaplan AL. Primary choriocarcinoma of the vulva. Int J Gynecol Cancer. 2001. 11:251–254.
12. Hammond CB, Schmidt HJ, Parker RT. McGowan L, editor. Gestational trophoblastic: disease. Gynecologic oncology. 1978. New York: Appleton-Century-Crofts;359–381.
13. Massenkeil G, Crombach G, Dominik S, De Bruyne F, Nitz U, Krussel J, et al. Metastatic choriocarcinoma in a postmenopausal woman. Gynecol Oncol. 1996. 61:432–437.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download