Abstract
Retroperitoneal teratoma with malignant transformation is a rare condition in adults. Its most common malignant transformation is into a squamous cell carcinoma, but rarely into a mucinous adenocarcinoma. Postoperative treatment of mucinous adenocarcinoma arising from teratomas has not been established due to its rare incidence. Here we present a case of retroperitoneal mucinous adenocarcinoma arising from a teratoma in the presacral area. Operative and postoperative managements are described with a brief review of the literatures.
Teratomas consist of elements from the ectoderm, endoderm, and mesoderm.1 Teratomas are found, in order of decreasing frequency, in the ovaries, testes, anterior mediastinum, retroperitoneal space, the presacral and coccygeal areas.2 Teratomas with malignant transformation (TMT) occurs in less than 1% of ovarian teratoma, most commonly developing into squamous cell carcinoma.3 Retroperitoneal TMT into a mucinous adenocarcinoma is extremely rare. Postoperative treatment of mucinous adenocarcinoma arising from teratomas has not been established due to its rare incidence. We report a case of a 45-year-old woman with mucinous adenocarcinoma arising from a retroperitoneal teratoma in the presacral area.
A 45-year-old premenopausal woman (gravida 2, para 2) suffering from anal pain for 2 months. On bimanual pelvic examination, an adult fist-sized fixed mass was palpated in the posterior cul de sac. According to her past history she had neither obvious medical disease nor surgery. Complete blood count and blood biochemistry were within normal limits. Serum carbohydrate antigen (CA) 19-9 was 607.9 U/ml. CA-125 was 64.91 U/ml. Colonoscopic findings were unremarkable except for external compression. Magnetic resonance image (MRI) demonstrated an 11×9 cm sized presacral multiseptated cystic mass with ill defined border around the sacrum (Fig. 1). This mass had irregular septal thickness with solid a growing portion, suggesting a retroperitoneal malignancy, such as a mucinous cystadenocarcinoma.
During laparotomy, an adult fist-sized well defined tumor was found in the presacral area. The peritoneum covering the tumor was intact and ascites was not seen. The uterus, both fallopian tubes and ovaries showed grossly normal appearance. There was adhesion suggesting tumor invasion between the tumor and presacral tissue. The tumor was ruptured during surgical resection. The tumor invasion involved the presacral venous plexus and the invasion led to intractable bleeding during dissection of the tumor from the presacral tissue. The bleeding was controlled by direct compression for 20 minutes. The resected mass was sent to a pathologist for frozen section analysis. The analysis showed a mucinous cystadenocarcinoma. We performed additional hysterectomy and bilateral salpingoophorectomy due to the possibility of metastasis from ovary.
Gross histopathologic findings showed a multilocular cystic mass which was partially lined by whitish gray mucosa with focal hemorrhage (Fig. 2). Microscopic findings revealed both mucinous adenocarcinoma and elements of mature teratoma (squamous epithelium, and ciliated pseudostratified columnar epithelium) (Fig. 3). There was also tumor invasion into the presacral muscle and periosteum. Therefore, it was diagnosed as a retroperitoneal mucinous adenocarcinoma arising from a teratoma. The patient had an unremarkable postoperative course. Postoperative concurrent chemoradiotherapy was administered with paclitaxel-cisplatin combination regimen. CA-125 and CA 19-9 became normal after chemoradiotherapy.
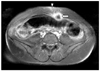
This patient complained of abdominal pain 8 months after surgery. Physical examination showed a hard mass with tenderness in the abdominal incision site. CA-125 and CA 19-9 levels were elevated to 41.77 U/ml and 85.30 U/ml, respectively. The MRI showed an ill defined solid tumor just beneath the abdominal skin incision site without signs of other recurrence (Fig. 4). A wide local excision of the abdominal wall mass was done and the histopathologic findings revealed a metastatic adenocarcinoma. At 13 months after the first operation, bowel resection and reanastomosis were performed due to intestinal obstruction. At 15 months, a 10 cm-sized cancer mass was found in the abdominal incision site. However, complete resection was impossible due to its broad extension along the abdominal wall. The patient expired from her disease at 19 months following the first operation.
Retroperitoneal teratoma accounts for only 6-11% of primary retroperitoneal tumors, and its incidence is bimodal with peaks in the first 6 months of life and in early adulthood.2 Ovarian TMT occurs in less than 1%, most commonly developing into a squamous cell carcinoma.3 Retroperitoneal TMTs are extremely rare in adults. In 1995, Tezel et al.4 stated that they had been able to find 10 cases reported since 1937. There have been only four reported cases of a retroperitoneal TMT into adenocarcinoma since 1995.5-7
Retroperitoneal TMT is usually identified only after it has grown into a huge mass. Definitive diagnosis of TMT is most often rendered postoperatively. Wang et al.7 reported that a reliable indicator of TMT is its extension to the adjacent structures and organs but not its contents. In this case, the MRI revealed findings of a mucinous tumor rather than those of a teratoma, and showed extension to the adjacent tissues. Fioretti et al.8 reported that the correlation of CA 19-9 with a mucinous ovarian tumor was significant and that CA 19-9 may be useful in monitoring patients with mucinous tumors. In this case, preoperative serum CA 19-9 level was elevated approximately eighteen times higher than the upper normal level and which normalized after the operation. Although this is only one case of retroperitoneal mucinous adenocarcinoma arising from a teratoma, it is consistent with the report of Fioretti et al.8 Because TMT tends to be chemoresistant and recurrence is common, the best treatment for long term survival is complete surgical resection. Although the prognostic factors of retroperitoneal TMT are presently not known, the poor prognostic factors of ovarian TMT include cyst wall invasion, rupture, tumor dissemination, ascites, adhesions, and adenocarcinoma.9 Based upon this, our patient showed poor prognostic factors including adhesion, adenocarcinoma and tumor rupture during operation.
In our case, during operation, frozen section analysis revealed a mucinous adenocarcinoma. We considered the possibility that the tumor had metastasized from the ovary. Therefore, we additionally performed a hysterectomy and bilateral salpingoophorectomy. Postoperative treatment of mucinous adenocarcinoma arising from teratoma has not been established due to its rare incidence. Roles of adjuvant therapy such as chemotherapy, radiotherapy, or concurrent chemoradiotherapy are still under investigation. Paclitaxel with cisplatin or carboplatin combination chemotherapy might be effective against mucinous adenocarcinomas because this combination chemotherapy is the regimen of choice in the postoperative management of ovarian epithelial cell carcinomas.10 We performed postoperative concurrent chemoradiotherapy with a paclitaxel-cisplatin combination regimen. The rationale to use concurrent chemoradiotherapy was that our patient had a locally advanced lesion with direct invasion to the presacral tissue, and the lesion was grossly confined in the pelvic cavity without visible intra-abdominal or distant metastatic lesions. In addition, radiation may have a curative benefit in a localized malignant tumor. With concurrent chemoradiation, local control of pelvis was successful, but metastasis to the abdominal wall was found 8 months after surgery. Metastatic spread of an adenocarcinoma to the skin and subcutaneous tissue may be by lymphatic and hematogenous spread, by direct extension or by implantation during surgery.11,12 In our case, the tumor recurred just beneath the abdominal skin incision site that did not receive the curative dose of radiation, and other metastatic foci were not found. It is likely that recurrence in this patient occurred by direct implantation due to cyst rupture during surgery.
We describe a case of patient with retroperitoneal mucinous adenocarcinoma arising from a teratoma in the presacral area. This is the first case report that concurrent chemoradiotherapy was used for treatment of a retroperitoneal TMT grossly confined to the pelvic cavity. This tumor is extremely rare, and there is no standard adjuvant therapy. Therefore, further clinical and pathological experience is needed for this rare tumor.
Figures and Tables
Fig. 1
MRI. Retroperitoneal cystic mass with multiple septations (T). Large bowel (arrow) is displaced upward by mass. This finding suggests its retroperitoneal location.
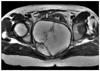
Fig. 2
Gross histopathologic findings show multilocular cystic mass that is partially lined by whitish gray mucosa and has focal hemorrhage.

References
1. Templeman CL, Fallat ME, Lam AM, Perlman SE, Hertweck SP, O'Connor DM. Managing mature cystic teratomas of the ovary. Obstet Gynecol Surv. 2000. 55:738–745.
2. Gschwend J, Burke TW, Woodward JE, Heller PB. Retroperitoneal teratoma presenting as an abdominal-pelvic mass. Obstet Gynecol. 1987. 70:500–502.
3. Renato F, Paolo V, Girolamo M, Vigano L, Alessandro P, Claudio V, et al. Malignant retroperitoneal teratoma: case report and literature review. Acta Urol Belg. 1996. 64:49–54.
4. Tezel E, Sare M, Edali N, Oguz M, Uluoglu O, Gokok NH. Retroperitoneal malignant teratoma: a case report. Mater Med Pol. 1995. 27:123–125.
5. Chu PY, Teng TH, Lee CC, Chou YY. Adenocarcinomas arising from primary retroperitoneal teratoma in an adult female patient. Int J Urol. 2006. 13:1352–1354.
6. Song ES, Choi SJ, Kim L, Choi SK, Ryu JS, Lim MK, et al. Mucinous adenocarcinoma arising from one retroperitoneal mature cystic teratoma in a postmenopausal woman. J Obstet Gynaecol Res. 2005. 31:127–132.
7. Wang LJ, Chu SH, Ng KF, Wong YC. Adenocarcinomas arising from primary retroperitoneal mature teratomas: CT and MR imaging. Eur Radiol. 2002. 12:1546–1549.
8. Fioretti P, Gadducci A, Ferdeghini M, Bartolini T, Bianchi R, Facchini V. Correlation of CA-125 and CA19-9 serum levels with clinical course and second-look findings in patients with ovarian carcinoma. Gynecol Oncol. 1987. 28:278–283.
9. Arora DS, Haldane S. Carcinosarcoma arising in a dermoid cyst of the ovary. J Clin Pathol. 1996. 49:519–521.
10. Ozols RF. Paclitaxel (Taxol)/carboplatin combination chemotherapy in the treatment of advanced ovarian cancer. Semin Oncol. 2000. 27(3):Suppl 7. 3–7.
11. Franciolini G, Momoli G, Minelli L, Mutolo F, Franchini MA, Chiodini S, et al. Cutaneous metastases from carcinoma of the cervix. Tumori. 1990. 76:410–412.
12. Lane G, Tay J. Port-site metastasis following laparoscopic lymphadenectomy for adenosquamous carcinoma of the cervix. Gynecol Oncol. 1999. 74:130–133.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download