Abstract
Objective
Platinum (Pt) based drugs including cisplatin and carboplatin are widely used as anticancer drugs in various human cancers. Many studies have shown that chemotherapeutic agents synergistically enhance cell death induced by death ligands. However it has been recently reported that cisplatin may inhibit tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced cell death through inactivation of caspases. Thus, we investigated whether carboplatin also inhibits TRAIL-induced cell death.
Methods
HeLa cells were treated with TRAIL in the presence of cisplatin or carboplatin, and cell death was analyzed using the crystal violet staining method. Caspase activation was checked through detection of Bid cleavage by Western blotting using anti-Bid antibody.
Platinum (Pt) drugs including cisplatin and carboplatin are widely used as anticancer drugs. Cisplatin was initially discovered as an inhibitor of cell division in bacteria by Barnett Rosenberg in 1965, and was found to have an anticancer effect in 1969, and approved for use as an anticancer drug in 1978.1 Since cisplatin is known to cause serious side effects, a second generation platinum-derived anticancer drug, carboplatin, was produced. Carboplatin has clinically been proven to have fewer side effects than cisplatin but shows similar activity.2,3 Both cisplatin and carboplatin are currently used to treat tumors of the head, neck, lungs, and genitourinary tract. Cisplatin and carboplatin cause DNA damage by forming DNA adducts leading to p53-dependent apoptotic cell death.4 Many studies have demonstrated that chemotherapeutic agents including cisplatin are able to synergistically enhance cell death induced by death ligands even in death ligand-resistant cells.5
The best characterized death ligands are tumor necrosis factor (TNF) family proteins including TNF-alpha, FasL, and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). TRAIL is a member of the TNF superfamily and a potent inducer of apoptosis. TRAIL triggers rapid cell death by interacting with two death receptors, DR4 and DR5, which contain the cytoplasmic death domains.6,7 TRAIL-induced death signaling is similar to FasL-induced death signaling. Ligand binding induces receptor trimerization. Fas-associated protein with death domain (FADD), and caspase-8 are recruited to form the death inducing signaling complex (DISC) complex. Activation of procaspase-8 at the DISC stimulates the downstream signaling cascades transmitted through two major pathways which are mitochondria-dependent and mitochondria-independent pathways.8 In the mitochondria-dependent pathway, activated caspase-8 cleaves Bid. The truncated Bid (tBid) translocates to the mitochondria leading to the loss of mitochondrial membrane potential, release of cytochrome c, activation of caspase-9, and the subsequent activation of caspase-3. The mitochondria-independent signaling pathway directly activates caspase-3 by activated caspase-8. In both pathways, activated caspase-3 cleaves target substrates and eventually leads to cell death.8
In a previous study, we showed that cisplatin inhibits TRAIL-induced cell death through inactivation of caspases.9 In this study, we confirmed that cisplatin inhibited TRAIL-induced cell death, but carboplatin showed no inhibitory effect on TRAIL-induced cell death.
Cisplatin (Choongwae Pharma Corporation, Seoul, Korea) and Carboplatin (Korea United Pharm, Inc, Seoul, Korea) were purchased. Recombinant active caspase-8 and pan-caspase inhibitor z-VAD-fmk were purchased from Calbiochem. Polyclonal anti-Bid antiserum was generated in rabbit using recombinant Bid protein as antigen.
HeLa cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 100 units/ml penicillin, and 100 µg/ml streptomycin. The cells were plated on 48-well plates for the cell death assay. Cell viability was determined by crystal violet staining as described.10 In brief, cells were stained with 0.4% crystal violet in methanol for 10 minutes at room temperature and then washed with tap water. Stained cells were extracted with 4% methanol, and dye extracts were measured at 540 nm wavelength using a microplate reader.
Recombinant active caspase-8 (100 ng) was pretreated with cisplatin (50 µg), z-VAD-FMK (0.1 mM), or carboplatin (50 µg), and incubated with recombinant Bid (100 ng) protein in caspase assay buffer (10 mM PIPES, 100 mM NaCl, 0.1 mM EDTA, 10 mM DTT, 10% sucrose, 0.1% CHAPS, pH 7.4) for 1 hour at 37℃. Cleavage of Bid was analyzed by Western blot with anti-Bid antibody.
A number studies have demonstrated that chemotherapeutic agents including cisplatin synergistically enhanced cell death by death ligands such as TRAIL, FasL, or TNF-alpha. However in a previous study, we showed that cisplatin inhibited death ligand-induced cell death.9 Thus, we first investigated whether or not carboplatin, a second generation analogue of cisplatin, also inhibits TRAIL-induced cell death. HeLa cells were treated with TRAIL for 24 hours in the presence of cisplatin or carboplatin, and the cell viability was analyzed by crystal violet staining. Cisplatin inhibited cell death by TRAIL, while carboplatin did not inhibit TRAIL-induced cell death in HeLa cells. Moreover, carboplatin treated HeLa cells were more sensitive to TRAIL than ordinary HeLa cells treated with TRAIL alone (Fig. 1A). The crystal violet-stained cells were extracted with 4% methanol, and dye extracts were measured at 540 nm wavelength using a microplate reader. Similar to the results in Fig. 1A, cisplatin had an inhibitory effect in a dose dependent manner, while carboplatin enhanced cell death in a dose dependent manner (Fig. 1B). These results suggest that carboplatin has no inhibitory effect on TRAIL-induced cell death inconsistent with cisplatin; however, carboplatin does sensitize the cells to TRAIL-induced cell death.
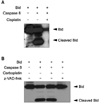
TRAIL induces the trimerization of the death receptors DR4/DR5 upon ligand binding. It subsequently recruits adapter proteins such as FADD and caspase-8 leading to the formation of DISC. The activated caspase-8 cleaves Bid to generate the truncated Bid (tBid) that translocates to mitochondria and causes mitochondrial dysfunction.8 Thus caspase-8-dependent Bid cleavage is a key mechanism in TRAIL-induced cell death. Our previous study showed that cisplatin inactivates caspases. To test whether carboplatin inhibits activation of caspase-8 or not, cleavage of recombinant Bid protein by caspase-8 was monitored by Western blotting. Consistent with previous results, cisplatin inactivated caspase-8 (Fig. 2A); however, carboplatin has no inhibitory effect on caspase-8 activation (Fig. 2B). These results suggest that carboplatin has no effect on caspase-8 activity unlike cisplatin.
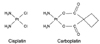
Cisplatin and carboplatin are widely used to treat various human cancers. These platinum-based chemotherapeutic drugs cause DNA adducts leading to cell death. They also bind to many extracellular and intracellular proteins, although they have different binding abilities.11,12 The formation of protein adducts with platinum-based drugs may be a key event for the tumor killing activities as well as side effects. The chemical structure of cisplatin is quite different from that of carboplatin, although both drugs contain platinum. Cisplatin contains a platinum atom complexed with two ammonia groups and two chloride residues, and the chloride residue in cisplatin interacts with the sulfur group of the cysteine residue forming a cisplatin-protein complex.11,13 Cisplatin interacts with caspases by direct binding of the chloride residue in cisplatin with the sulfur group of the cysteine residue in caspases and lead to the inactivation of the caspases due to formation of a complex of cisplatin and caspase.9 However, the two chlorides in cisplatin have been substituted with 1,1-cyclobutane-dicarboxyl residue in carboplatin (Fig. 3), possibly removing the functional moiety for binding to caspases. Based on the fact that carboplatin has no inhibitory effect on TRAIL-induced cell death, we speculate that carboplatin does not directly interact with caspase-8. These results provide evidence that carboplatin functions differently from cisplatin.
Figures and Tables
Fig. 1
Cisplatin inhibits TRAIL-induced cell death in HeLa cells, but not carboplatin. Cisplatin (100, 200 µg/ml) or carboplatin (100, 200 µg/ml) was incubated with/without TRAIL (100 ng/ml) for 24 hours in HeLa cells. (A) The cells were stained with Crystal violet. (B) Cell viability was measured by methanol extraction after crystal violet staining of the cells.
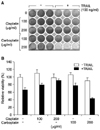
Fig. 2
Cisplatin directly inhibits activity of caspase-8, but not carboplatin. (A) Pretreated recombinant human caspase-8 (100 ng) with cisplatin (250 µg) was incubated with recombinant Bid (100 ng) in 50 µl of caspase assay buffer for 1 hour at 37℃. (B) Pretreated recombinant human caspase-8 (100 ng) with carboplatin (250 µg) in the presence or absence of pan-caspase inhibitor zVAD-FMK was incubated with recombinant Bid (100 ng) in 50 µl of caspase assay buffer for 1 hour at 37℃. Cleavage of Bid was analyzed by Western blot with anti-Bid antibody.
References
1. Rosenberg B, Vancamp L, Krigas T. Inhibition of cell division in escherichia coli by products from a platinum electrode. Nature. 1965. 205:698–699.
2. Harrap KR. Preclinical studies identifying carboplatin as a viable cisplatin alternative. Cancer Treat Rev. 1985. 12:Suppl A. 21–33.
3. Smith IE, Harland SJ, Robinson BA, Evans BD, Goodhart LC, Calvert AH, et al. Carboplatin: a very active new cisplatin analog in the treatment of small cell lung cancer. Cancer Treat Rep. 1985. 69:43–46.
4. Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003. 22:7265–7279.
5. Munshi A, McDonnell TJ, Meyn RE. Chemotherapeutic agents enhance TRAIL-induced apoptosis in prostate cancer cells. Cancer Chemother Pharmacol. 2002. 50:46–52.
6. Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997. 276:111–113.
7. Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, et al. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997. 16:5386–5397.
8. Seol DW, Li J, Seol MH, Park SY, Talanian RV, Billiar TR. Signaling events triggered by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL): caspase-8 is required for TRAIL-induced apoptosis. Cancer Res. 2001. 61:1138–1143.
9. Shin JN, Seo YW, Kim M, Park SY, Lee MJ, Lee BR, et al. Cisplatin inactivation of caspases inhibits death ligand-induced cell death in vitro and fulminant liver damage in mice. J Biol Chem. 2005. 280:10509–10515.
10. Kim YM, Kim TH, Seol DW, Talanian RV, Billiar TR. Nitric oxide suppression of apoptosis occurs in association with an inhibition of Bcl-2 cleavage and cytochrome c release. J Biol Chem. 1998. 273:31437–31441.
11. Ivanov AI, Christodoulou J, Parkinson JA, Barnham KJ, Tucker A, Woodrow J, et al. Cisplatin binding sites on human albumin. J Biol Chem. 1998. 273:14721–14730.
12. Mandal R, Kalke R, Li XF. Interaction of oxaliplatin, cisplatin, and carboplatin with hemoglobin and the resulting release of a heme group. Chem Res Toxicol. 2004. 17:1391–1397.
13. Dedon PC, Borch RF. Characterization of the reactions of platinum antitumor agents with biologic and nonbiologic sulfur-containing nucleophiles. Biochem Pharmacol. 1987. 36:1955–1964.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download