Abstract
Malignant transformation of a mature cystic teratoma (MCT) is an uncommon complication. The most common form of malignant transformation of a MCT is squamous cell carcinoma, representing 75% of malignant transformations. The frequency of malignant transformation of MCT to adenocarcinoma is just 6.8%. To the best of our knowledge, no case of para-aortic lymph node metastasis in mucinous adenocarcinoma arising from MCT has been reported before. The prognosis of malignant transformation of the MCT is very poor. Here, we report an unusual case of a 41-year-old woman with mucinous adenocarcinoma arising from MCT with para-aortic lymph node metastasis.
The mature cystic teratoma (MCT) is the most common germ cell tumor of ovary, composing more than 20% of all ovarian neoplasms and occurring at any age, with a peak incidence in the first two decades of life. Malignant transformation of a MCT is an uncommon complication occurring in approximately 1-3% of all mature cystic teratomas.1 Although any of the constituent tissues of teratoma has the potential to undergo malignant transformation, squamous cell carcinoma is the most commonly associated cancer.2 Other tumors arising in a MCT include adenocarcinoma, basal cell carcinoma, adenosquamous carcinoma, thyroid carcinoma, sebaceous carcinoma, malignant melanoma, sarcoma, carcinoid tumor, and neuroectodermal tumor.2-4 To the best of our knowledge, there is no report so far that has presented para-aortic lymph node metastasis in mucinous adenocarcinoma arising from a MCT. We describe the case with unusual occurrence of mucinous adenocarcinoma arising from a MCT with para-aortic lymph node metastasis as her only evidence of extraovarian disease, which was treated with staging operation and postoperative chemotherapy.
A 41-year-old multiparous woman (gravida 2, para 2) was referred to our hospital from a local hospital with intermittent lower abdominal pain and an abdominal palpable mass, beginning one month prior to her visit. In the initial physical examination, an approximately adult head-sized movable cystic mass with regular contour was detected in the left lower abdomen. The uterus and right adnexa were not palpable due to the large mass. The cervix was of normal appearance. There was no induration and no nodular mass of the Douglas pouch. The rectal examination was free. Otherwise her physical examination was unremarkable. Pelvic ultrasonogram showed 13×10 cm sized thickened, multiseptated solid-cystic mass with calcification in the left ovary. The uterus and the right ovary were unremarkable. Pelvic computed tomography showed a 15×9 cm sized multiseptated solid-cystic mass with internal calcification and fatty component in the left ovary (Fig. 1). There was also para-aortic lymph node enlargement and ascites. There were no significant abnormal findings in the colon or small intestine. Papanicolaou smear was normal. Routine laboratory investigations revealed no abnormalities. Chest radiograph and electrocardiogram were within normal limits. The value of serum carcinoembryonic antigen (CEA) was 41.8 ng/ml and that of CA 125 was 45 U/ml. The endometrial biopsy showed late secretary phase. A gastroduodenoscopy and colon study to exclude other primary cancers metastatic to the ovary were normal. Exploratory laparotomy was performed.
On entering the peritoneal cavity, the left ovary was found to be a over adult- head sized semisolid cystic mass with an intact smooth capsule. The frozen biopsy at the operating room disclosed a mucinous cystadenocarcinoma combined with benign cystic teratoma. The left para-aortic lymph node revealed firm and thumb-tip sized enlargement. Hysterectomy with bilateral salpingo-opherectomy, appendectomy, omentectomy, pelvic and para-aortic lymph node dissection, and peritoneal lavage were performed.
Grossly the left ovarian tumor measured 16×16×10 cm and weighed 1247 gm. It was multilocular mass with an intact smooth capsule. The cut surface of the left ovarian mass disclosed multiseptated myxoid and mucinous areas, and Rokitansky's protubernace composed of osteocartilagenous tissue and hair tufts and mixed yellow sebaceous material. The ovarian surface was clear (Fig. 2A). The pathologic diagnosis was mucinous adenocarcinoma arising from a mature cystic teratoma of the left ovary. The cystic portion of the ovary consisted of well differentiated mucinous adenocarcinoma, which was intimately associated with dermoid tissue mature cystic teratoma (Fig. 2B). The tumor cells of the well differentiated (more than 90%) mucinous adenocarcinoma were positive for cytokeratin 20 and negative for cytokeratin 7, suggesting primary ovarian mucinous tumor that must have arisen in the colonic type epithelium of the mature cystic teratoma (Fig. 3A). The left para-aortic lymph nodes showed metastasis from poorly differentiated adenocarcinoma (Fig. 3B). The tumor cells were positive for cytokeratin 7 and some of them were positive for cytokeratin 20, suggesting that the tumor originated from the solid area of the ovarian lesion. The peritoneal cytology was negative for malignancy. There was also a mature cystic teratoma in the right ovary and no recognized abnormality in the appendix. Neoplastic cells were not seen in the extraovarian sites, except for the left para-aortic lymph node.
Her postoperative course was not eventful. Eight days after the operation, adjuvant combination chemotherapy per 3 weeks using paclitaxel (135 mg/m2) - carboplatin (75 mg/m2) - gemcitabine (700 mg/m2) was administered nine times. Postoperatively, upon the completion of the first chemotherapy, the values of CA 125 and CEA returned to normal. Currently, at 39 postoperative months, the patient is well and disease-free. She was given pelvic examinations, vaginal cytology, and tumor marker follow-up every 3 months and abdomino-pelvic computered tomography follow-up annually.
Mature cystic teratomas (MCT) are recognized as one of the most common tumors in women during the reproductive age. Malignant change in benign cystic teratoma has been recorded as occurring in 1-3% of cases.1 The most common form of malignant transformation of the MCT is squamous cell carcinoma. Other tumors arising in MCT include basal cell carcinoma, sebaceous tumor, malignant melanoma, adenocarcinoma, sarcoma, and neuroectodermal tumor. The risk of malignancy is related to age and is substantially greater in postmenopausal women, the highest incidence being in the fifth and sixth decades of life.5
Malignant change is rarely recognized preoperatively. Most patients with such tumors have symptoms which do not differ from those of a uncomplicated mature cystic teratoma. Adenocarcinoma arising from a benign MCT is extremely rare. Because of the rarity of these tumors, few studies have been able to make a preoperative diagnosis. Definitive diagnosis is most often rendered postoperatively.
Mucinous ovarian tumors occasionally may be associated with MCT and the mucinous epithelium in such cases may be histologically benign, borderline or malignant.6-11 It is now well-established that cytokeratin (CK) 7/ CK 20 expression profiles are quite useful for distinguishing primary ovarian mucinous tumors from metastases of the lower intestinal tract origin (appendix, colorectum), as most often exhibit diffuse expression of CK 20 coupled with lack of or limited expression of CK 7, whereas primary gastrointestinal tract mucinous tumors secondarily involving the ovaries most often exhibit diffuse expression of CK 7 coupled with variable expression of CK 20 that is often present, but usually patchy rather than diffuse.12,13 In our case the mucinous epithelium was diffusely immnuoreactive for CK 20 and CEA, and negative for CK 7. This result suggested that the tumor has originated from the solid area of the ovarian lesion. Our patient also had a raised preoperative serum CA125 and CEA level, similar to the findings in the previously reported case.14 To the best of our knowledge, though there are several reports about occurrence of a mucinous adenocarcinoma arising from a MCT, there is no report that has presented para-aortic lymph node metastasis in such cases so far. We describe the case with unusual occurrence of mucinous adenocarcinoma arising from MCT with para-aortic lymph node metastasis as her only evidence of extraovarian disease.
The optimal management of mucinous cystadenocarcinoma arising from MCT has not been established. In our case, the adjuvant combination chemotherapy per 3 weeks using gemcitabine (700 mg/m2) carboplatin (75 mg/m2) paclitaxel (135 mg/m2) was administered because gemcitabine carboplatin paclitaxel combination chemotherapy is effective in the postoperative management of advanced ovarian carcinoma.15 The prognosis of patients with malignant transformation in MCT is very poor, with most women dying within one year. Occasional cases with prolonged survival have been reported.16 Poor prognostic factors include tumor dissemination, cyst wall invasion, ascites, spontaneous or accidental rupture, adhesion, and tumor type other than squamous carcinoma.17 In this current case, at 39 postoperative months, the patient is well and disease-free. She was given pelvic examinations, vaginal cytology, and tumor markers follow-up every 3 months and abdomino-pelvic computed tomography follow-up annually.
We present a unusual case of mucinous adenocarcinoma arising from a MCT with para-aortic lymph node metastasis as her only evidence of extraovarian disease which was treated with staging operation and postoperative chemotherapy.
Figures and Tables
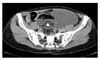 | Fig. 1Pelvic CT shows a 15×9 cm sized multiseptated solid-cystic mass with internal calcification and fatty component in the left ovary. |
 | Fig. 2(A) Macroscopic appearance of ovarian tumor shows muliseptated myxoid and mucinous areas and Rokitansky's protubernace composed of osteocartilagenous tissue and hair tufts admixed yellow sebaceous material. The ovarian surface is clear. (B) The cystic portion of the ovary consists of well differentiated mucinous adenocarcinoma (inset; H&E, ×200), which is intimately associated with dermoid tissue mature cystic teratoma (H&E, ×100). |
 | Fig. 3(A) The tumor cells of the well differentiated mucinous adenocarcinoma is positive for cytokeratin 20 (left lower) and negative for cytokeratin 7 (right lower), suggesting the mucinous tumor must have arisen in the colonic type epithelium of the matue cystic teratoma. (B) The left para-aortic lymph node shows metastasis from poorly differentiated adenocarcinoma. The tumor cells are positive for cytokeratin 7 (left lower) and some of them are positive for cytokeratin 20 (right lower), suggesting that the tumor has originated from the solid area of the ovarian lesion (H&E, ×100). |
References
1. Griffiths D, Wass J, Look K, Sutton G. Malignant degeneration of a mature cystic teratoma five decades after discovery. Gynecol Oncol. 1995. 59:427–429.
2. Rose PG, Tak WK, Reale FR. Squamous cell carcinoma arising in a mature cystic teratoma with metastasis to the paraaortic nodes. Gynecol Oncol. 1993. 50:131–133.
3. Krumerman MS, Chung A. Squamous carcinoma arising in benign cystic teratoma of the ovary: A report of four cases and review of the literature. Cancer. 1977. 39:1237–1242.
4. Curling OM, Potsides PN, Hundson CN. Malignant change in benign cystic teratoma of the ovary. Br J Obstet Gynaecol. 1979. 86:399–402.
5. Gordon A, Rosenshein N, Parmley T, Bhagavan B. Benign cystic teratomas in postmenopausal women. Am J Obstet Gynecol. 1980. 138:1120–1123.
6. Lee KR, Scully RE. Mucinous tumors of the ovary: A clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with "pseudomyxoma peritonei". Am J Surg Pathol. 2000. 24:1447–1464.
7. McKenna JC, Soslow RA, Longacre TA. Mucinous neoplasms arising in mature cystic teratomas: A clinicopathologic study of ovarian and sacrococcygeal tumors. Mod Pathol. 2004. 17:206A.
8. Tang P, Soukkary S, Kahn E. Mature cystic teratoma of the ovary associated with complete colonic wall and mucinous cystadenoma. Ann Clin Lab Sci. 2003. 33:465–470.
9. Hunter V, Barnhill D, Jadwin D, Crooks L. Ovarian mucinous cystadenocarcinoma of low malignant potential associated with a mature cystic teratoma. Gynecol Oncol. 1988. 29:250–254.
10. Fishman A, Edelstein E, Altaras M, Beyth Y, Bernheim J. Adenocarcinoma arising from the gastrointestinal epithelium in benign cystic teratoma of the ovary. Gynecol Oncol. 1998. 70:418–420.
11. Fujiwara K, Ginzan S, Silverberg SG. Mature cystic teratomas of the ovary with intestinal wall structures harboring intestinal-type epithelial teratomas. Gynecol Oncol. 1995. 56:97–101.
12. Cathro HP, Stoler MH. Expression of cytokeratins 7 and 20 in ovarian neoplasia. Am J Clin Pathol. 2002. 117:944–951.
13. Vang R, Gown AM, Barry TS, Wheeler DT, Yemelyanova A, Seidman JD, et al. Cytokeratins 7 and 20 in primary and secondary mucinous tumors of the ovary: Analysis of coordinate immunohistochemical expression profiles and staining distribution in 179 cases. Am J Surg Pathol. 2006. 30:1130–1139.
14. Stewart CJ, Tsukamoto T, Cooke B, Leung YC, Hammond IG. Ovarian mucinous tumour arising in mature cystic teratoma and associated with pseudomyxoma peritonei: Report of two cases and comparison with ovarian involvement by low-grade appendiceal mucinous tumour. Pathology. 2006. 38:534–538.
15. Fuso L, Amant F, Neven P, Berteloot P, Vergote I. Gemcitabine-carboplatin-paclitaxel combination as first-line therapy in advanced ovarian carcinoma: A single institution phase II study in 24 patients. Int J Gynecol Cancer. 2006. 16:Suppl 1. 60–67.
16. Ueda G, Fujita M, Ogawa H, Sawada M, Inoue M, Tanizawa O. Adenocarcinoma in a benign cystic teratoma of the ovary: Report of a case with a long survival period. Gynecol Oncol. 1993. 48:259–263.
17. Arora DS, Haldane S. Carcinosarcoma arising in a dermoid cyst of the ovary. J Clin Pathol. 1996. 49:519–521.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download