Abstract
Multiple primary cancer is defined as the multiple occurrence of malignant neoplasms in the same individual. Due to the development of new diagnostic techniques and the rise in long-term survival of cancer, reports of multiple primary cancers have gradually increased. Herein, we describe the case of a 68-year-old female patient with quadruple primary cancer of the breast, rectum, ovary, and endometrium. For its great rarity, we report this case with a review of the literature.
Multiple primary cancer is defined as two or more cancers with no subordinate relationship occurring either simultaneously or not in the same patient. The incidence of multiple primary cancer is 2~6.3% of all cancers. Most are double primary cancers, and the incidence decreases as the number of concomitant cancers increases. The incidence of quadruple primary cancer has been reported as <0.1%.1 Multiple primary cancer of the female reproductive tract represents 1-6%. Ayhan et al.2 reported that the incidence of multiple primary cancer of the female reproductive tract is 1.7%, and 51.7% of these cases occur in the endometrium and ovary. Moertal et al.3 reported that the incidence of cancer in additional organs in females with reproductive tract cancer is 2.8%, and the most frequent pairings are ovarian cancer and lung cancer, or ovarian cancer and colon cancer. We describe here an extremely rare case of quadruple primary cancer involving synchronous ovarian and endometrial cancer that occurred after breast and rectal cancer. We also reviewed the relevant literature.
A 68-year-old woman (para 3-0-7-3) being followed-up by the general surgery department of Samsung Medical Center was referred to the obstetrics and gynecology department after a right ovarian cyst was detected on abdomen-pelvis CT. The patient had received radical mastectomy 23 years ago for breast cancer, and that cancer had not recurred. The patient visited the internal medicine clinic of another hospital five years ago due to general weakness. A colonoscopy performed in the outpatient department found a rectal polyp, and the ensuing biopsy revealed moderately differentiated adenocarcinoma of the rectum. The patient was transferred to the general surgery department of Samsung Medical Center for additional work-up and treatment four years ago on July 28th, 2004. Abdomen-pelvis CT revealed no special abnormalities, but transrectal ultrasonography showed a polyp limited to the submucosal layer of the rectum, and TEM (transanal endoscopic microsurgery) was performed. A polypoid specimen of about 1.5×1.2 cm was obtained.
The pathology report showed moderately to poorly differentiated adenocarcinoma of the rectum (Fig. 1). There was a high risk of recurrence and lymph node metastasis, so lower anterior resection (LAR) was performed after a month. The pathology report described no evidence of malignancy in the obtained colon and no metastasis in the 12 perirectal lymph nodes. The tumor was staged as 1 (T1N0M0), and the patient was followed as an outpatient with no further treatment. An abdomen-pelvis CT performed 41 months later revealed a 6 cm-sized complex cyst that seemed to originate from the right ovary, and the patient was referred to the obstetrics and gyne cology department. Past medical history was unexceptional, except as listed above. Among family members, her father was diagnosed with cancer at about age 70, but she could not remember what kind of cancer. Out of five siblings, one brother and one sister were diagnosed with colon cancer at about age 60. The patient did not remember her age of menarche, and menopause occurred at age 47. Initial vital signs were blood pressure of 131/79 mmHg, pulse rate 82/min, and body temperature 36℃. Physical examination was nonspecific except for a mass of 5-6 cm palpated in the right pelvis.
In the laboratory investigation, CA-125 was 12.7 IU/ml. Pelvis ultrasonography showed hydrometra 4.6 mm thick in the endometrium (Fig. 2A). A 7.8×6 cm-sized complicated cyst was seen in the right adnexa, and the left adnexa was not observed (Fig. 2B). The abdomen- pelvis CT revealed a 6 cm-sized complicated cyst in the right adnexa (Fig. 2C). An endometrial aspiration biopsy was performed due to the hydrometra, but the specimen was not appropriate for diagnosis.
Exploratory laparotomy was performed due to the right ovary tumor. There was a 5×4 cm-sized solid and cystic mass originating from the right ovary and containing brownish fluid and friable necrotic tissue adhesed to the uterus and posterior cul de sac. The uterus and left ovary were atrophied, and looked grossly normal. No grossly metastatic lesions were found in the rest of the abdominal cavity. There was no gross evidence of recurrent rectal cancer. A total abdominal hysterectomy, bilateral salpingoophorectomy, bilateral pelvic lymph node dissection, and infracolic omentectomy were performed. Microscopically, the tumor of the right ovary showed complex papillary structures with irregular branching and tufting (Fig. 3). Histopathologic diagnosis was grade II serous adenocarcinoma extending to the mesoovarium. FIGO stage was IIc. Concurrently, the endometrium showed irregular papillary structures with thin fibrovascular cores lined by high grade tumor cells (Fig. 4A). Most areas of the tumor were confined within the endometrium, but focally superficial myometrial invasion was identified of stage Ib and Grade II. The background of the endometrium demonstrated multifocal serous intraepithelial carcinoma (Fig. 4B, arrow) that displayed strong immunoreactivity with anti-p53 antibody (1:50, Santacruz Biotechnology, CA, USA) (Fig. 4B, inset, arrow). There were no pathologic findings such as signet ring cells suggestive of a Krukenberg tumor. Further study, including immunohistochemistry for CK-7 and CK-20, was not performed. After surgery, the patient was started on Taxotere/Carboplatin combination chemotherapy, which she is currently continuing.
Since Billroth4 first reported a case of tumors in multiple organs in 1889, the occurrence of multiple primary cancer has risen as the cure rate and survival increase due to improvements in diagnostic techniques and treatment. Multiple primary cancer is defined as two or more cancers that do not have any subordinate relationship and develop independently. The diagnostic criteria for multiple primary cancer remains the same criteria advocated by Warren and Gates5 in 1932. Namely: 1) Each cancer must be definitively malignant by histopathology, 2) they must be histologically different, and 3) the possibility of metastasis among the cancers must be excluded.
It is not easy to differentiate between multiple primary and multicentric cancers with this criteria, however, and therefore Moertel et al.6 suggested a classification including multiple primary cancers and multicentric cancers, and this classification is used widely today. Group I includes multiple primary cancers occurring in organs with the same histology, group II includes multiple primary cancers originating from different tissues, and group III consists of cancers from different tissues and organs that concurrently exist with group I, forming multiple primary cancer of three or more cancers. Group I is further subdivided into group A, including cancers that occur in the same tissue and organ, group B, including cancers that are from the same tissue and different organs, and group C, including cancers that occur in bilateral organs (i.e., breast, ovary, etc.).
Moertel et al.3 classified multiple primary cancers observed at the same time or within six months as synchronous multiple primary cancers, and cancers developing with more than six months as an interval as metachronous multiple primary cancers.
The mechanism of multiple primary cancers is not fully known, but many hypotheses have been suggested, such as family history, immunologic and genetic defects, prolonged exposure to carcinogens, radiation and chemotherapy for the primary cancer, and field cancerization.
Field cancerization is a concept that was suggested by Slaughter et al.7 in 1953. It suggests that when the body is exposed to carcinogens, other organs besides the organ with cancer are also exposed to the carcinogen and carry a high risk of cancer. Goodall et al.8 hypothesized that the female upper reproductive system and the peritoneal epithelium have the same origin. Laughlan et al.9 suggested that cancers developing in other sites originate from histologically similar epithelium. This "secondary mullerian system" concept attempts to explain the etiology of endometrial and ovarian synchronous multiple primary cancers.2 Recently, Iioka et al.10 observed that patients with multiple primary cancers have a high frequency of microsatellite instability (MSI), arguing that MSI affects the pathogenesis of some multiple primary cancers.
The incidence of multiple primary cancers appears to be rising. Close to a century after Billroth et al. reported three cases of multiple primary cancers in 1889, Cunliffe et al.11 reported the incidence of multiple primary cancers as 10.7% in 1984. In Korea, the incidence of multiple primary cancers was reported as 0.35% in 1970 by Kim et al.12, 0.74% in 1984 by Yun et al.13, and 1.43% in 1999 by Koo et al.14
There are no established therapeutic rules for multiple primary cancers, but the type, progression, response to therapy, and patient's general health status should be considered. If each of cancers has the possibility for cure, radical therapy is indicated. If radical therapy of the primary cancer is impossible, conservative therapy is indicated for the secondary cancer.
In this case of quadruple cancers of the breast, rectum, ovary, and endometrium, the carcinoma of the rectum was pathologically composed of large tubuloglandular structures lined by intestinal epithelium, whereas the serous carcinoma of the ovary and endometrium showed micropapillary architecture lined by a non-intestinal type of epithelium. The ovarian tumor displayed unilateral involvement, which is a rare finding in metastatic ovarian tumors. The endometrial carcinoma was mostly confined within the endometrium with background serous intraepithelial carcinomas showing characteristic p53 immunoreactivity. Although a number of candidate cancer genes have been analyzed in serous carcinoma, only the p53 tumor suppressor gene has been shown to be significantly altered, with mutations identified in almost 90% of cases.15,16 In fact, there are few other tumor types that demonstrate the mutation frequency in a single gene as high as that of p53 in serous endometrial carcinoma. Furthermore, approximately 75% of endometrial intraepithelial carcinomas, the putative precursor of serous carcinoma, have mutations in p53.16 All of these findings strongly suggest that the cancers of the rectum, ovary, and endothelium were independent primary tumors in the described case.
In addition, multiple cancers involving the intestinal tract, breast, endometrium, and ovary may be associated with hereditary cancer syndromes. In particular, the main cancer susceptibility syndromes that involve gynecologic cancers, namely Hereditary Breast-Ovarian Cancer Syndrome (HBOC) and Hereditary Nonpolyposis Colorectal Cancer Syndrome (HNPCC), may be associated with this case. HBOC affects more individuals than any other hereditary cancer syndrome,17 and is caused by germ line mutations in BRCA1 and BRCA2, autosomal tumor suppressor genes. Development of breast cancer at an early age is one of the strongest predictors of the presence of HBOC. Individuals at high risk can be identified by their personal history of ovarian or early-onset breast cancer in women or breast cancer in men, and/or by a family history of these cancers in either the paternal or maternal lineages.18
HNPCC is the second most common hereditary cancer syndrome, accounting for 3-5% of all colorectal cancers and 2-3% of all endometrial cancers. The disease is caused by germ line mutations in one of five DNA mismatch repair genes: MSH2, MLH1, MSH6, PMS2, and PMS1. Early-onset colorectal cancer and/or endometrial cancer are the strongest predictors of the disorder, particularly when they are seen together in an individual or family, are accompanied by other tumors associated with the disease, and occur in more than one generation of the family. Another predictor of HNPCC is the presence of MSI in the DNA of the colorectal cancer.
Our patient had breast cancer at age 45, rectal cancer at age 63, and ovarian cancer and endometrial cancer synchronously at age 68. Although none of her family members had breast or ovarian cancer, genetic study for HBOC (BRCA1,2) was warranted because she had breast cancer at an early age (<age 50) and epithelial ovarian cancer. Because of her synchronous ovarian and endometrial cancer development after rectal cancer, HNPCC cannot be excluded, in spite of her older age at onset (>age 50) of the rectal cancer. So, in this case, we cannot exclude the possibility of HNPCC or HBOC. Unfortunately, we could not perform the genetic tests for HBOC (BRCA1,2) and HNPCC (MSH2, MLH1) because the patient refused them. We suspect that the pathogenesis of multiple primary cancers may be related to genetic factors or family history in this case.
Multiple primary cancer is rarely reported in Korea, but it is believed that the incidence is rising. Prevention, early diagnosis, and treatment will become important, and further studies are required.
Figures and Tables
 | Fig. 1Endoscopic mucosal resection of the rectal polyp showing a moderate to poorly differentiated adenocarcinoma with submucosal invasion (hematoxylin and eosin ×40). |
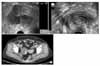 | Fig. 2(A) Pelvis ultrasonography of a 7.8×6.0 cm-sized, multiseptated, solid portion-containing mass arising from the right ovary. (B) Pelvis ultrasonography of hydrometra of 3.6 mm thickness. (C) Abdominopelvic CT showing a 6 cm-sized cyst. |
References
1. Nakayama H, Masuda H, Ugajin W, Nakamura Y, Akiyama K, Suzuki K, et al. Quadruple cancer including bilateral breasts, Vater's papilla, and urinary bladder: Report of a case. Surg Today. 1999. 29:276–279.
2. Ayhan A, Yalçn OT, Tuncer ZS, Gügan T, Küçüali T. Synchronous primary malignancies of the female genital tract. Eur J Obstet Gynecol Reprod Biol. 1992. 45:63–66.
3. Moertel CG, Dockerty MB, Baggenstoss AH. Multiple primary malignant neoplasm: II. Tumors of different tissues or organs. Cancer. 1961. 14:231–237.
4. Billroth T. General surgical pathology and therapeutics in 51 Vorlesungen: A textbook for students and physicians in fifty-one lectures. 1889. 14th ed. Berlin, DE: G. Rerimer.
5. Warren S, Gates O. Multiple primary malignant tumors; Surgery of literature and statistical study. Am J Cancer. 1932. 16:1358–1414.
6. Moertel CG. Multiple primary malignant neoplasms: Historical perspectives. Cancer. 1977. 40:Suppl 4. 1786–1792.
7. Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; Clinical implications of multicentric origin. Cancer. 1953. 6:963–968.
8. Goodall J. The origin of epithelial new growths of the ovary. Surg Gynecol Obstet. 1911. 14:584.
9. Lauchlan SC. Conceptual unity of the Mülerian tumor group: A histologic study. Cancer. 1968. 22:601–610.
10. Iioka Y, Tsuchida A, Okubo K, Ogiso M, Ichimiya H, Saito K, et al. Metachronous triple cancers of the sigmoid colon, stomach, and esophagus: Report of a case. Surg Today. 2000. 30:368–371.
11. Cunliffe WJ, Hasleton PS, Tweedle DE, Schofield PF. Incidence of synchronous and metachronous colorectal carcinoma. Br J Surg. 1984. 71:941–943.
12. Kim CK, Chang JW. Multiple primary malignant tumors. J Korean Surg Soc. 1970. 12:63–71.
13. Yun HK, Kim JB. Multiple primary malignant neoplasm. J Korean Surg Soc. 1984. 26:1–9.
14. Koo DJ, Yun DS, Lee JJ, Park CJ. Clinical analysis of 65 multiple primary cancer cases. J Korean Surg Soc. 1999. 56:137–142.
15. Moll UM, Chalas E, Auguste M, Meaney D, Chumas J. Uterine papillary serous carcinoma evolves via a p53-driven pathway. Hum Pathol. 1996. 27:1295–1300.
16. Tashiro H, Isacson C, Levine R, Kurman RJ, Cho KR, Hedrick L. p53 gene mutations are common in uterine serous carcinoma and occur early in their pathogenesis. Am J Pathol. 1997. 150:177–185.
17. Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004. 4:665–676.
18. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology - Genetic/familial high-risk assessment: Breast and Ovarian. 2005. Fort Washington: National Comprehensive Cancer Network.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download