Abstract
Objective
To evaluate the value of sonographic morphology indexing (MI) system and serum CA-125 levels in the assessment of the malignancy risk in patients with ovarian tumors.
Methods
From September 2000 to July 2006, 202 patients who underwent surgery for ovarian tumors were reviewed retrospectively. In all patients, the MI score and serum CA-125 level were measured preoperatively. The association of the final pathologic diagnosis with the MI score and serum CA-125 level were examined.
Results
There were 26 malignant tumors out of 141 ovarian tumors with a MI ≥5 (18%). With a cut-off value of 5, the sensitivity, specificity, PPV, and NPV of MI scores were 0.743, 0.293, 0.181, and 0.845, respectively. There were 22 malignant tumors out of 54 ovarian tumors with serum CA-125 >30 u/ml (41%). With a cut-off value of 30 u/ml, the sensitivity, specificity, PPV, and NPV of serum CA-125 level were 0.667, 0.808, 0.407, and NPV 0.925, respectively. On ROC curve, the optimal cut-off value of MI score was 6.5-7.5 and that of serum CA-125 level was 25.6-28.5 u/ml. With a cut-off value of 7, the sensitivity and 1-specificity of MI score were 0.875-0.917 and 0.023-0.203, respectively. After the exclusion of teratoma cases, the sensitivity and 1-specificity of MI score were 0.875-0.917 and 0.046-0.138, respectively. With a cut-off value of 25.6-28.5 u/ml, the sensitivity and 1-specificity of serum CA-125 level were 0.958 and 0.203-0.215, respectively.
In Korea, ovarian cancer accounts for 3.6% of cancers which occurs in women and is the eighth common cancer. Among gynecologic cancers, ovarian cancer is the second most common cancer and its incidence is rapidly increasing. For example, the number of women with a newly-diagnosed ovarian cancer increased from 461 in 1991 to 1572 in 2002.1
More than half of epithelial ovarian cancers which accounts for almost 90% of ovarian cancers are detected in the advanced stage.2 The suggested reasons why so many ovarian cancers are detected in the advanced stage are the lack of symptoms in early stage and the absence of effective screening programs.3 Because early ovarian cancer has a better prognosis than advanced disease, there have been continuous efforts to identify early ovarian cancer.
Transvaginal sonography (TVS) is a sensitive method for detecting ovarian tumors. However, the positive predictive value (PPV) of TVS in diagnosing ovarian cancer is relatively low.4 Especially, teratomas are frequently misdiagnosed as ovarian cancer by TVS because teratomas have diverse sonographic features such as a cystic tumor, a complex echogenic tumor with internal solid portions, a tumor consisting of fat, or a tumor with calcifications.5 However, the incidence of cancer among teratomas is only 3%, and over 95% of teratomas are known to be a benign tumor.6 Therefore, the diagnosis of ovarian cancer based on sonographic examination alone may be misleading.
In addition to sonography, serum CA-125 level is one of the important tools to differentiate a malignant tumor from a benign ovarian tumor because the serum CA-125 level is elevated in over 80% of patients with ovarian cancer.7
The preoperative estimation of malignancy risk in ovarian tumors became an important issue in gynecology because of the widespread use of laparoscopy in gynecologic surgery. Despite of the widespread use of laparoscopy, there were some problems associated with laparoscopic surgery. For example, delay in surgical staging and intraoperative tumor spillage were reported in 13-100% of cases where unexpected cancer was recognized after a laparoscopic surgery. In addition, the effect of laparoscopic surgery on prognosis in patients with ovarian cancer who underwent the laparoscopic surgery is still unclear.8
The objective of this study was to evaluate the value of sonographic morphology indexing (MI) system and serum CA-125 levels in the estimation of the malignancy risk in patients with ovarian tumors.
We performed a retrospective review of patients with ovarian tumors who underwent surgery at our institute between September 2000 and June 2006. The indications for surgery was the presence of a postmenopausal ovarian tumor, an ovarian tumor which is larger than 5 cm and increasing in size over several months, a newly appearing or persistent ovarian cyst while taking oral pills, an ovarian tumor larger than 10 cm, a solid ovarian tumor with a possibility of ovarian cancer, a tumor with solid portions or papillary projections, or an ovarian tumor with suspicious metastasis. Only patients who had a definite pathologic diagnosis and were not treated due to medical problems within six months were included; patients with borderline ovarian tumors were excluded.
Using the 3.5 MHz transvaginal probe of Accuvix XQ (Medison, Seoul, Korea) and Aloka SSD 1700 (Aloka, Tokyo, Japan), the size of the ovarian tumors was measured three-dimensionally. The volume of the ovarian tumor was calculated under the assumption that the shape of ovarian tumor is an ellipsoid (volume = x × y × z × 0.523). Ovarian tumors whose volumes were greater than 10 cm3 in postmenopausal women and 20 cm3 in premenopausal women were considered as abnormal.9 In addition, the presence of solid portions or papillary projections was noted for ovarian cysts.10
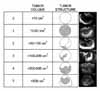
MI score was calculated by adding the score of the volume (0-5) to the score of the structure (0-5) according to the method of Ueland et al.10 (Table 1). Specifically, the septum structure which Depriest et al.8 had included into the MI system was removed from the MI system by Ueland et al.10 because the association of septum structure with the malignancy risk of ovarian tumors was weak. Instead, the internal echogenecity of tumor and external fluid collection were added to the MI system (Fig. 1).
For pathologically proven teratomas, tumors were classified into four categories by sonographic findings: a cyst (A), a tumor with echogenic materials (sebaceous gland, sebum, or hair) without a cystic portion (B), a tumor with highly echogenic materials (bone, teeth) without a cystic portion (C), a tumor with highly echogenic materials with a cystic portion (D).11
Preoperative serum CA-125 levels were measured in all patients. Laparoscopic oophorectomy or tumorectomy was performed for 114 patients and laparotomy was performed for 88 patients. Thirty-five patients were diagnosed as having ovarian cancer by frozen section examination and underwent hysterectomy, bilateral adnexectomy, retroperitoneal lymphadenectomy, and peritonectomy.
The association of the pathologic diagnosis with the MI score and serum CA-125 levels were examined in mucinous or serous tumors, separately. Tumors were classified according to the WHO classification and stage was determined according to the FIGO system.
The cut-off values of MI score and serum CA-125 levels to differentiate a malignant tumor from a benign tumor were determined using the ROC curves. The association of the pathologic diagnosis with the MI score and serum CA-125 levels were evaluated with the paired student's t-test. In addition, the association of the pathologic diagnosis with the MI score and serum CA-125 levels was evaluated using the chi-square test after the MI score and serum CA-125 levels were converted into categorical variables using the cut-off values. p values smaller than 0.05 was considered to be statistically significant and all statistical analyses were performed using SPSS ver. 12 (SPSS inc., Chicago, USA).
The mean age of patients was 39.4 years (SD=15.4; range 11-86). Among 202 patients, 167 patients had a benign ovarian tumor and 35 patients had a malignant ovarian tumor (Table 2). Out of 35 patients with a malignant tumor, 25 patients (71%) had stage 1 disease; 1 patient (3%) had stage 2 disease; six patients (17%) had stage 3 disease; and three patients (9%) had stage 4 disease.
The MI score was associated with the pathologic diagnosis (Table 3). A malignant tumor was diagnosed in 9 out of 61 cases with an MI score <5 (15%) and in 26 out of 141 cases with an MI score ≥5 (18%) (p=0.686). With a cut-off value of 5, the sensitivity, specificity, PPV, and negative predictive value (NPV) of the MI score was 0.743, 0.293, 0.181, and 0.845, respectively. In serous tumors, the sensitivity, specificity, PPV, and NPV of the MI score was 0.571, 0.633, 0.421, and 0.760, respectively. In mucinous tumors, the sensitivity, specificity, PPV, and NPV of the MI score was 0.875, 0.792, 0.737, and 0.905, respectively.
On ROC curves, the MI score 6.5-7.5 was the most ideal cut-off value with a sensitivity of 0.875-0.917 and 1-specificity of 0.023-0.203. A malignant tumor was diagnosed in 15 out of 144 cases with an MI score <7 (10%) and in 20 out of 58 cases with an MI score ≥7 (36%) (p<0.001). With a cut-off value of 7, the sensitivity, specificity, PPV, and NPV of the MI score was 0.571, 0.754, 0.328, and 0.893, respectively (Fig. 2). In serous tumors, the sensitivity, specificity, PPV, and NPV of the MI score was 0.357, 0.800, 0.455, and 0.727, respectively. In mucinous tumors, the sensitivity, specificity, PPV, and NPV of the MI score was 0.750, 0.917, 0.857, and 0.846, respectively. The MI score was associated with the pathologic diagnosis in mucinous tumors (p<0.001) but not in serous tumors (p=0.095).
Out of 89 mature teratomas, nine tumors were cysts (A), 52 tumors were tumors with echogenic materials (sebaceous gland, sebum, or hair) without a cystic portion (B), 22 tumors were tumors with highly echogenic material (bone, teeth) without a cystic portion (C), and six tumors were tumors with highly echogenic materials with a cystic portion (D) (Fig. 3). Eighty out of 89 mature teratomas had an MI score equal to or higher than 5. Many mature teratomas had solid portions on sonographic examination. After excluding cases with mature teratomas with a cut-off value of 7, the sensitivity and 1-specificity of the MI score was 0.875-0.917 and 0.046-0.138, respectively. The specificity of the MI score was greater when the cases with mature teratomas were excluded from the analysis (Fig. 4).
Serum CA-125 level was associated with the pathologic diagnosis (p=0.006) (Table 4). Eleven out of 146 patients whose serum CA-125 ≤30 u/ml (7%) and 22 out of 54 patients whose serum CA-125 >30 u/ml (41%) were diagnosed as having a malignant tumor (p<0.001). With a cut-off value of 30 u/ml, the sensitivity, specificity, PPV, and NPV of serum CA-125 level was 0.667, 0.808, 0.407, and 0.925, respectively. In serous tumors, the sensitivity, specificity, PPV, and NPV of serum CA-125 level was 0.642, 0.733, 0.500, and 0.192, respectively. In mucinous tumors, the sensitivity, specificity, PPV, and NPV of serum CA-125 level was 0.813, 0.333, 0.650, and 0.850, respectively.
In serous and mucinous tumors, the serum CA-125 levels of patients with a malignant tumor was compared with that of patients with a benign tumor using the student t-test. In serous tumors, the serum CA-125 levels of patients with a malignant tumor was different from that of patients with a benign tumor (p=0.045). However, in mucinous tumors, the serum CA-125 level of patients with a malignant tumor was not different from that of patients with a benign tumor (p=0.057).
On ROC curve, serum CA-125 level 25.6-28.5 u/ml was the ideal cut-off value with a sensitivity of 0.958 and 1-specificity of 0.203-0.215. With a cut-off value of 27 u/ml, the sensitivity, specificity, PPV, and NPV of serum CA-125 level was 0.697, 0.826, 0.442, and 0.929, respectively (Fig. 2). Seventeen out of 30 patients (57%) whose MI score was ≥7 and serum CA-125 >27 u/ml were diagnosed as having a malignant tumor. Via the chi-square test, the sensitivity of this combination strategy (MI score+serum CA-125) was higher than that of the MI score alone or serum CA-125 level alone (p<0.001).
The estimation of malignancy risk in patients with ovarian tumors is important considering the improved survival of patients with early ovarian cancer, which was reported in several recent studies.12 The most effective diagnostic tool should be accurate, easy to perform, and cheap.13 Furthermore, it should be helpful in determining the order of treatment for high-risk patients and in deciding the extent and time of surgery for low-risk patients.14
Several studies have evaluated the value of the sonographic MI system in the estimation of malignancy risk in ovarian tumors.6,13,14 To differentiate a malignant tumor from a benign tumor, the MI system minimizes the inter-observer variability and maximizes the interpretability of sonographic examinations.15 In this study, the MI system employed was invented by Depriest et al.8 and was later modified by Ueland et al.10 Ueland et al. removed the septum structure from the system because the association of septum structure with pathologic diagnosis was weak. Instead, Ueland et al. added the structure of wall, volume of tumor, general echogenecity, and external fluid collection to the MI system. After modification by Ueland et al., the sensitivity, specificity, PPV, and NPV of the MI system improved. In this study, a malignant tumor was diagnosed in nine out of 61 cases with an MI score <5 (15%), and in 26 out of 141 cases with an MI score ≥5 (18%) (p=0.686). Baily et al.16 performed surgery on 45 patients with a simple ovarian cyst smaller than 5 cm, and reported that no malignant tumor was diagnosed. In another study, no malignant tumor was detected during the follow-up of 86 patients with simple cysts smaller than 5 cm. Therefore, an ovarian cyst can be followed-up with regular sonographic examinations unless the ovarian cyst is a complex cyst with solid portions or septums.16 In this study, no malignant tumor was diagnosed among 21 simple cysts smaller than 5 cm. However, in complex tumors with an MI score ≥5, 18% of tumors were identified as a malignant tumor. After the exclusion of cases with mature teratomas, 50% of tumors were malignant tumors. Considering that surgery is rarely performed for a simple ovarian cyst and long-term follow-up is difficult for patients with a simple cyst, a large-scale study would be necessary to ascertain long-term prognosis of a simple ovarian cyst.
Most mature teratomas can be diagnosed by sonographic examination.17 Sonographic features of mature teratomas are as follows: an echogenic nodule projecting into the internal side of cyst which is also known as a mural nodule or a Rokitansky nodule,18 an echogenic tumor with sound attenuation which are produced by the sebum or hair in the cyst, and a tumor with internal thin echogenic bands which are produced by the hair in the cyst.17 In this study, mature teratomas were classified into four categories by sonographic findings: a cyst (A), a tumor with echogenic materials (sebaceous gland, sebum, or hair) without a cystic portion (B), a tumor with highly echogenic materials (bone, teeth) without a cystic portion (C), a tumor with highly echogenic materials with a cystic portion (D) (Fig. 3). Meis et al. reported that the sensitivity of sonography in diagnosing mature teratomas was 58% and the specificity was 99%.11 In this study, 80 out of 85 mature teratomas had an MI score equal to or higher than 5 and this high MI score of mature teratomas increased the false positive rate of the MI score. By exclusion of mature teratomas, with the cut-off value of 7, the specificity of the MI score increased from 0.754 to 0.862-0.954. Therefore, in ovarian tumors which do not look like mature teratomas, an MI score equal to or higher than 7 was the ideal cut-off value to differentiate a malignant tumor from a benign tumor.
Although it is difficult to make a strong conclusion, we suggest that the sonographic features of a malignant tumor can be different from those of a mature teratoma by classifying the sonographic features of mature teratomas into four categories.
The immature teratomas had non-specific sonographic features such as a mixed-echogenic tumor with solid portions.17 A sonographic examination is accurate in the diagnosis of a benign teratoma but not in the diagnosis of a malignant teratoma. Therefore, additional studies such as magnetic resonance imaging (MRI) are necessary to diagnose an immature teratoma.19 According to Grab et al.,20 sonography had a higher sensitivity than MRI or positron emission tomography (PET) in differentiating a malignant tumor from a benign ovarian tumor, although the specificity of the sonography was low. In addition, Kurz et al.21 reported that MRI was superior to sonography or computed tomography (CT) for the detection of ovarian tumors, but all three imaging studies had a similar accuracy in the staging of cancer and the differentiation of a malignant tumor from a benign tumor. Therefore, performing all three imaging studies on ovarian tumors would be more sensitive in the detection of a malignant tumor than performing one or two studies. However, the benefits of performing all three imaging studies is still unclear considering the financial burden and delay of surgery.22 Therefore, the accuracy of sonography in the differentiation of a malignant tumor from a benign tumor may be increased by employing the MI system after the exclusion of teratomas.
Serum CA-125 levels are also helpful in the differentiation of a malignant tumor from a benign ovarian tumor. Jacobs et al.reported that over 80% of patients with ovarian cancer had a serum CA-125 level higher than 30 u/ml, and the sensitivity and specificity of serum CA-125 level measurement was 81% and 75% with the cut-off value of 30 u/ml.7 In this study, after the exclusion of immature teratomas and mixed germ cell tumors, 11 out of 146 cases (7%) with serum CA-125 ≤30 u/ml and 22 out of 54 cases (41%) with serum CA-125 >30 u/ml were diagnosed as a malignant tumor (p<0.001). With a cut-off value of 27 u/ml, the sensitivity and specificity of serum CA-125 level were 70% and 83%, respectively. In postmenopausal women with serum CA-125 >200 u/ml, the PPV of serum CA-125 level was 96%.23 Visintin et al.24 reported that the accuracy of the combination method which included measurements of CA-125, leptin, prolactin, macrophage inhibiting factor (MIF), osteoponin, and IGF-II levels for the diagnosis of ovarian cancer reached 98.7%. Therefore, measurement of serum CA-125 levels is thought to be helpful for the estimation of the malignancy risk when a malignant tumor is suspected by sonographic examination. However, the false negative rate of serum CA-125 level is high in small volume tumors. In addition, the specificity of serum CA-125 level in premenopausal women is low because the serum CA-125 level can be elevated in several benign diseases.23 Therefore, a large-scale, prospective study would be necessary to clarify the role of serum CA-125 level in ovarian tumors.
Because this study was undertaken in a referral hospital, many cases of ovarian cancers and solid ovarian tumors were included in this study. Therefore, both the MI score ≥7 and serum CA-125 >30 u/ml were associated with pathologic diagnosis in mucinous tumors but not in serous tumors (in mucinous tumors; p<0.001 for the MI score, p=0.045 for serum CA-125 level). A larger-scale study including other types of ovarian cancers would be necessary to evaluate the values of the MI score and serum CA-125 level.
Preoperative MI score and serum CA-125 level measurements are relatively accurate methods to estimate the malignancy risk in ovarian tumors. Recently, laparoscopic surgery is being widely performed for the treatment of ovarian tumors.25 The safety of laparoscopic surgery for ovarian tumors is still unclear because of possible complications such as intraoperative cyst rupture, spillage of cyst contents, chemical peritonitis, and unexpected malignant tumor.25 However, for a benign ovarian tumor, a laparoscopic surgery is considered to be safe because complications related with a cyst rupture is rare.25 Therefore, the preoperative estimation of malignancy risk in ovarian tumors is important to determine the treatment plan and type of surgery. The estimation of malignancy risk in ovarian tumors can be more accurate by measuring the MI score and serum CA-125 level.
Figures and Tables
Fig. 2
The ROC curve including ovarian teratomas. The cutoff range is a MI 6.5-7.5. It has a sensitivity 0.875-0.917 and 1-specificity 0.023-0.203. The cutoff range is CA-125 25.60-28.50. It has a sensitivity 0.958 and 1-specificity 0.203-0.215. MI: morphology indexing.

Fig. 3
Four sonographic findings of ovarian teratomas. Four types are a cystic pattern (A), a dense echo pattern with or without a cystic component (B), a pattern including a dense echogenic component with or without a cystic component (C), a densely echogenic tubercle associated with a cystic echo pattern (D).
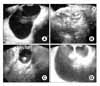
Fig. 4
The ROC curve excluding ovarian teratomas. A cutoff range is a MI 6.5-7.5. It has a sensitivity 0.875-0.917 and a 1-specificity 0.046-0.138. MI: morphology indexing.

References
1. Ministry of Health and Welfare Republic of Korea, Korea Central Cancer Registry. 2002 Annual report of the Korea central cancer registry (2002.1.1-2002.12.31). 2003. Seoul: Ministry of Health and Welfare Republic of Korea.
2. Katsube Y, Berg JW, Silverberg SG. Epidemiologic pathology of ovarian tumors: A histopathologic review of primary ovarian neoplasms diagnosed in the Denver Standard Metropolitan Statistical Area, 1 July-31 December 1969 and 1 July-31 December 1979. Int J Gynecol Pathol. 1982. 1:3–16.
3. Korean Society of Obstetrics and Gynecology. Annual report of gynecologic cancer registry program in Korea (2003.1.1-2003. 12.31). 2006. Seoul: Jin press.
4. van Nagell JR Jr, DePriest PD, Reedy MB, Gallion HH, Ueland FR, Pavlik EJ, et al. The efficacy of transvaginal sonographic screening in asymptomatic women at risk for ovarian cancer. Gynecol Oncol. 2000. 77:350–356.
5. Togashi K. MR imaging of the ovaries: normal appearance and benign disease. Radiol Clin North Am. 2003. 41:799–811.
6. Scully RE, Young RH, Clement RB. Tumors of the ovary, maldeveloped gonads, fallopian tube, & broad ligament - 1998. 1998. Washington, DC: Armed Forces Institute of Pathology.
7. Jacobs I, Oram D, Fairbanks J, Turner J, Frost C, Grudzinskas JG. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br J Obstet Gynaecol. 1990. 97:922–929.
8. DePriest PD, Shenson D, Fried A, Hunter JE, Andrews SJ, Gallion HH, et al. A morphology index based on sonographic findings in ovarian cancer. Gynecol Oncol. 1993. 51:7–11.
9. Pavlik EJ, DePriest PD, Gallion HH, Ueland FR, Reedy MB, Kryscio RJ, et al. Ovarian volume related to age. Gynecol Oncol. 2000. 77:410–412.
10. Ueland FR, DePriest PD, Pavlik EJ, Kryscio RJ, van Nagell JR Jr. Preoperative differentiation of malignant from benign ovarian tumors: The efficacy of morphology indexing and Doppler flow sonography. Gynecol Oncol. 2003. 91:46–50.
11. Mais V, Guerriero S, Ajossa S, Angiolucci M, Paoletti AM, Melis GB. Transvaginal ultrasonography in the diagnosis of cystic teratoma. Obstet Gynecol. 1995. 85:48–52.
12. Partridge EE, Barnes MN. Epithelial ovarian cancer: Prevention, diagnosis, and treatment. CA Cancer J Clin. 1999. 49:297–320.
13. DePriest PD, Varner E, Powell J, Fried A, Puls L, Higgins R, et al. The efficacy of a sonographic morphology index in identifying ovarian cancer: A multi-institutional investigation. Gynecol Oncol. 1994. 55:174–178.
14. Sassone AM, Timor-Tritsch IE, Artner A, Westhoff C, Warren WB. Transvaginal sonographic characterization of ovarian disease: Evaluation of a new scoring system to predict ovarian malignancy. Obstet Gynecol. 1991. 78:70–76.
15. Higgins RV, van Nagell JR Jr, Woods CH, Thompson EA, Kryscio RJ. Interobserver variation in ovarian measurements using transvaginal sonography. Gynecol Oncol. 1990. 39:69–71.
16. Bailey CL, Ueland FR, Land GL, Depriest PD, Gallion HH, Kryscio RJ, et al. The malignant potential of small cystic ovarian tumors in women over 50 years of age. Gynecol Oncol. 1998. 69:3–7.
17. Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: Tumor types and imaging characteristics. Radiographics. 2001. 21:475–490.
18. Quinn SF, Erickson S, Black WC. Cystic ovarian teratomas: The sonographic appearance of the dermoid plug. Radiology. 1985. 155:477–478.
19. Choi JH, Tong SY, Kim MJ, Park JS, Lim YT, Kim JH, et al. Preoperative MR imaging for differentiation of immature from mature ovarian teratomas. Korean J Obstet Gynecol. 2006. 49:1547–1553.
20. Grab D, Flock F, Stohr I, Nussle K, Rieber A, Fenchel S, et al. Classification of asymptomatic adnexal masses by ultrasound, magnetic resonance imaging, and positron emission tomography. Gynecol Oncol. 2000. 77:454–459.
21. Kurtz AB, Tsimikas JV, Tempany CM, Hamper UM, Arger PH, Bree RL, et al. Diagnosis and staging of ovarian cancer: Comparative values of Doppler and conventional US, CT, and MR imaging correlated with surgery and histopathologic analysis--report of the Radiology Diagnostic Oncology Group. Radiology. 1999. 212:19–27.
22. Royal College of Obstetricians and Gynaecologists. Ovarian cysts in postmenopausal women (Guideline; no. 34). 2003. London: Royal College of Obstetricians and Gynaecologists.
23. Korean Society of Obstetrics and Gynecology. Gynecology. 2007. 4th ed. Seoul: Ko-Rye Press.
24. Visintin I, Feng Z, Longton G, Ward DC, Alvero AB, Lai Y, et al. Diagnostic markers for early detection of ovarian cancer. Clin Cancer Res. 2008. 14:1065–1072.
25. Mecke H, Savvas V. Laparoscopic surgery of dermoid cysts: Intraoperative spillage and complications. Eur J Obstet Gynecol Reprod Biol. 2001. 96:80–84.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download