Abstract
Objective
To verify whether it can be justified to classify patients to stage IIIC epithelial ovarian cancer based on nodal involvement only.
Methods
This study included all consecutive patients with stage IIIC epithelial ovarian cancer who underwent upfront cytoreductive surgery according to the FIGO guideline followed by platinum based chemotherapy from September 1989 to September 2006 at Asan Medical Center.
Results
During the study period, a total of 272 patients met the inclusion criteria. Optimal cytoreduction was achieved in 213 patients, and complete cytoreduction was achieved in 85 patients. Median follow-up time was 37 months (range, 6-181 months). The 5-year disease free survival (DFS) and overall survival (OS) rate of all patients were 23% and 57%, respectively. Forty-one patients were allocated to stage IIIC by positive nodes only. Patients with stage IIIC disease due to positive nodes only had significantly longer DFS and OS compared to other stage IIIC patients (p<0.001 and p<0.001). The DFS and OS of these patients was significantly better than those of other stage IIIC patients who achieved complete or optimal cytoreduction (p<0.001 and p<0.001). The outcome was even better than that of stage IIIA and IIIB patients (p<0.05 and p<0.05).
Ovarian cancer represents 25% of all malignancies in the female genital tract and is the most common cause of death among women who develop gynecologic malignancies in the western countries.1 It is the second most common gynecologic malignancy in Korea, with 1,300 new cases estimated to occur each year.2 It is the 8th most common female cancer and 9th most common cause of female cancer death in Korea.2 One of the major problems with regard to ovarian cancer is the fact that the majority (nearly 75%) of patients present with advanced stage disease.3 Initial comprehensive surgical staging according to the International Federation of Gynecology and Obstetrics (FIGO) staging systems with maximal cytoreductive surgery followed by taxene/platinum-based adjuvant chemotherapy is now considered to be a standard treatment for patients with ovarian cancer.4,5 Despite initial high response rates to first-line treatment, the majority of patients with ovarian cancer ultimately develop recurrent tumors and require further treatment.6
Lymph node metastasis in ovarian cancer has been reported to be an important prognostic factor,7-9 and thus the FIGO staging systems for ovarian cancer have included lymph node status since 1987.4,10 According to the FIGO staging system, patients with lymph node metastasis, even if the primary tumor is confined to the ovary or pelvis, are enrolled to stage IIIC.4 However, FIGO stage IIIC disease is a heterogenous group including two different extents of disease; 1) disease with lymph node metastasis regardless of the extent of intraperitoneal tumor implantation, and 2) disease with intraperitoneal tumor implants greater than 2 cm outside the pelvis.4 Although the extent of intraperitoneal tumor implants has been reported to be associated with prognosis of patients with ovarian cancer,11-14 the prognostic role of lymph node metastasis has not been determined. It is not clear if prognosis of patients with stage IIIC disease solely by virtue of lymph node metastasis is equivalent to that of patients with stage IIIC disease by virtue of large abdominal implants. The aim of this study was to verify whether it can be justified to classify patients to stage IIIC ovarian cancer based on nodal involvement only.
Patients with ovarian cancer who were treated and followed from Sep 1989 to Sep 2006 at Asan Medical Center (AMC; Seoul, Korea) were identified from a computerized database and cancer registry. This study included all consecutive patients with previously untreated stage IIIC epithelial ovarian cancer who underwent upfront cytoreductive surgery according to FIGO guidelines followed by platinum based chemotherapy. Patients who did not receive primary treatment or follow-up care at the AMC, who did not undergo complete staging procedure, who have histologic types other than epithelial ovarian cancer, or who have received neoadjuvant chemotherapy were excluded. Demographic data obtained from each patient's medical records included age, menopausal status, and parity, as well as the patient's histories of cancer, medical disease, and surgery or radiation therapy on the pelvis. We also obtained clinical data on the presence of tumor markers; the outcomes of surgical treatments; the presence of ascites; the presence of residual tumor cells after surgery; the histologic type, size, and grade of tumors; peritoneal cytology; lymph node involvement; surgical stage, as determined using FIGO system for ovarian cancer; adjuvant therapy; recurrence; and treatment at the times of recurrence and death. Clinicopathological prognostic factors and treatment outcomes were analyzed. AMC does not require approval from the Institutional Review Board for retrospective chart reviews; hence this analysis was exempt from the approval process.
Cytoreductive surgery was defined as optimal if the largest dimension of the largest residual tumor (RT) measured ≤1.0 cm, and suboptimal if it measured >1.0 cm. Overall survival (OS) time was calculated as the number of months from the date of surgery to either the date of death or the date censored. Disease-free survival (DFS) time was calculated as the number of months from the date of surgery to either the date of recurrence or the date censored. Survival curves and rates were calculated using the Kaplan-Meier method.15 Differences in survival were assessed using the log-rank test for categorical factors16 and Cox's proportional hazards model for continuous factors in univariate analysis.17 A multivariate analysis was performed using Cox's proportional hazards model to determine the survival benefit when the model was adjusted for favorable prognostic variables. Stepwise model-selection methods were used to select factors for inclusion in the multivariate Cox proportional hazards model. Frequency distributions were compared using Chi-squared and Fisher's exact tests, and mean and median values between groups were compared using a Student's T-test and the Mann-Whitney U-test. A p-value of less than 0.05 in a two-sided test indicated a significant difference. Data were analyzed using SPSS ver. 11 (SPSS Inc., Chicago, IL, USA).
During the study period, a total of 272 patients with stage IIIC epithelial ovarian cancer met the inclusion criteria. The characteristics of 272 patients are shown in Table 1. The mean age of all patients was 58 years (range, 22 to 81 years). Of these, 164 patients were postmenopausal, 197 patients had serous type tumors, and 122 patients had grade 3 tumors. Initial CA 125 level was elevated in 260 patients. At initial cytoreductive surgery, 149 patients had ascites ≥1,000 ml. Optimal cytoreduction was achieved in 213 patients, and complete cytoreduction was achieved in 85 patients. All patients received adjuvant chemotherapy with platinum based regimen and taxane was included in 194 patients. Of these, 104 patients received more than 6 cycles of chemotherapy.
Median follow-up time was 37 months (range, 6 to 181 months). The 5-year DFS and OS rate of all patients were 23% and 57%, respectively, and the 10 year DFS and OS rate were 19% and 38%, respectively (Fig. 1). In univariate analysis, patients who underwent optimal cytoreduction and who received adjuvant chemotherapy including taxane had longer DFS (Table 2, Fig. 2). DFS was not influenced by patient age, menopause, parity, initial CA 125 level, presence of ascites >1,000 ml, histologic type of tumor, grade of tumor, or number of chemotherapy cycles (Table 2). Patients who were older than 50 years of age, who underwent optimal cytoreduction and who received adjuvant chemotherapy including taxane had longer OS (Table 2, Fig. 2). OS was not influenced by menopause, parity, initial CA 125 level, presence of ascites >1000 ml, histologic type of tumor, grade of tumor, or number of chemotherapy cycles (Table 2). In multivariate analysis, DFS was significantly influenced by optimal cytoreduction, and OS was significantly influenced by age and adjuvant chemotherapy including taxane (Table 3).
Forty-one patients were allocated to stage IIIC for positive nodes only. Of these, 8 had disease limited to the ovaries and 33 had disease confined to the pelvis. Patients with stage IIIC disease due to positive nodes only had significantly longer DFS and OS compared to other stage IIIC patients (p<0.001 and p<0.001)(Fig. 3). The DFS and OS of these patients was significantly better than that of other stage IIIC patients who achieved complete or optimal cytoreduction (p<0.001 and p<0.001)(Fig. 3). The survival of patients with stage IIIC disease were compared with that of 34 patients with stage IIIA-IIIB disease who were treated and followed at AMC during the same period. The outcome was even better than that of stage IIIA and IIIB patients (p<0.05 and p<0.05)(Fig. 4).
In our series, 16 patients had rare histologic types of epithelial ovarian cancer (6 transitional cell carcinomas, 4 clear cell carcinomas, 4 undifferentiated carcinomas, and 1 squamous cell carcinoma). Of these, seven patients (2 clear cell carcinomas, 3 transitional cell carcinomas, 1 undifferentiated carcinoma, and 1 squamous cell carcinoma) had recurrent disease after a median disease free interval of 23 months (range, 1 to 79 months), and three patients (one each of clear cell carcinoma, transitional cell carcinoma, and undifferentiated carcinoma) died of disease after a median follow-up time of 30 months (range, 1 to 79 months).
Comprehensive surgical staging according to FIGO guideline is the mainstay treatment of epithelial ovarian cancer. Up to 10-25% of patients with presumable early stage disease which is confined to the ovaries or pelvis are upstaged after thorough surgical staging procedure due to pelvic lymph node metastasis.18-21 Paraaortic lymph nodes were positive in 6% of these patients.19 Because lymph node involvement is an important prognostic factor,7-9,22 patients with metastasized lymph nodes is assigned stage IIIC even if their intraperitoneal disease is equivalent to stage I-IIIB according to the FIGO staging system for ovarian cancer.4,10 However, the prognosis of patients who were enrolled to stage IIIC solely by lymph node involvement has not yet been clarified.
Some previous studies suggested that the prognoses of stage IIIC patients who are upstaged based on lymph node positivity are as poor as those of patients with stage IIIC based on intraperitoneal tumor spread.7,23,24 However, recent studies suggest that patients with stage IIIC disease by node involvement only have a more favorable prognosis than other stage IIIC disease patients.25-27 Onda et al.27 analyzed 103 ovarian cancer patients in stage I-III who underwent optimal cytoreductive surgery with systematic aortic and pelvic lymphadenectomy at initial surgery to investigate whether systematic aortic and pelvic lymphadenectomy would affect the prognoses. In their series, 14 patients who were upstaged to stage IIIC based on lymph node positivity had better 5 year survival than stage IIIC patients who had intraperitoneal tumors beyond the pelvis, irrespective of lymph node status (84% vs. 26%, p=0.042). Kanazawa et al.25 analyzed 117 patients with stage I-III epithelial ovarian cancer and reported that the survival was significantly worse in the node-positive group compared to that in the node-negative group for stage I-IIIB disease (p=0.0212), and that patient survival of patients with node-positive stage I-IIIB disease was significantly better than that of stage IIIC disease of abdominal implants greater than 2 cm in diameter outside the pelvis (p<0.0001). Cliby et al.26 analyzed 115 patients with stage IIIC epithelial ovarian cancer to describe the clinical behavior of occult stage IIIC. In their series, thirty-six patients were upstaged to stage IIIC by virtue of positive nodes and sixty nine patients were classified as stage IIIC disease because of obvious abdominal disease larger than 2 cm. In their series, the outcomes of occult stage IIIC were superior to other stage IIIC cytoreduced to either no gross residual disease or residual disease <1 cm, who had large volume upper abdominal disease at beginning of surgery (p<0.001). In our study, we demonstrated that patients with stage IIIC epithelial ovarian cancer due to positive nodes only had a more favorable prognosis than other stage IIIC disease. These findings are consistent with previous reports.25-27 However, the better survival of patients upstaged to stage IIIC by positive lymph nodes compared to patients with other stage IIIC could simply reflect the prognostic impact of small versus large tumor size.28 Therefore, a comparison between stage IIIC solely by lymph node metastasis and stage IIIA/IIIB patients would be more appropriate and provide evidence about possible differences in the biological and clinical behavior of lymph node versus peritoneal metastasis.29 In our series, stage IIIC patients solely by lymph node metastasis showed even better outcomes than stage IIIA and IIIB patients.
In our series, OS time was calculated as the number of months from the date of surgery to either the date of death or the date censored and DFS time was calculated as the number of months from the date of surgery to either the date of recurrence or the date censored. However, there were differences in the completeness of debulking of tumor, number of cycles of adjuvant chemotherapy, and response to adjuvant chemotherapy. These factors should be considered in the interpretation of survival outcomes. In our series, optimal cytoreduction was a significant factor for overall survival in univariate analysis. After adjusting for other significant factors such as age and use of taxene, odds ratio for death in patients who underwent optimal cytoreduction was 0.7 (95% confidence interval=0.5-1.1). Although it did not reach statistical significance, there is a trend of improved overall survival in patients who underwent optimal cytoreduction.
In conclusion, the results of our study suggest that the assignment to stage IIIC by lymph node involvement in ovarian cancer may be overestimating the risk of recurrence and death in these patients. Therefore, a large prospective study to reevaluate the role of lymph node metastasis in stage IIIC epithelial ovarian cancer is required.
Figures and Tables
Fig. 1
DFS (left) and overall survival OS (right) of 272 patients with stage IIIC epithelial ovarian cancer.

Fig. 2
DFS (left) and OS (right) of 272 patients with stage IIIC epithelial ovarian cancer by largest dimension of largest residual tumor at the completion of primary cytoreductive surgery. RD: largest dimension of largest residual tumor at the completion of primary cytoreductive surgery.
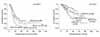
Fig. 3
DFS (left) and OS (right) of 272 patients with stage IIIC epithelial ovarian cancer. Patients with stage IIIC disease due to positive nodes only had significantly longer DFS and OS compared to other stage IIIC patients (p<0.001 and p<0.001). The DFS and OS of these patients was significantly better than that of other stage IIIC patients who achieved complete or optimal cytoreduction (p<0.001 and p<0.001).

Fig. 4
DFS (left) and OS (right) of 306 patients with stage III epithelial ovarian cancer. The outcome of patients with stage IIIC disease due to positive nodes only was even better than that of stage IIIA and IIIB patients.

References
1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006. 56:106–130.
2. Korean Society of Obstetrics and Gynecology. Annual report of gynecologic cancer reistry program in Korea for 2004 (2004.1.1-2004.12.31). Korean J Obstet Gynecol. 2007. 50:28–78.
3. Young RC, Fuks Z, Hoskins WJ. Devita VT, Hellman S, Rosenberg SA, editors. Cancer of the ovary. Cancer: Principles and practice of oncology. 1989. 3rd ed. Philadelphia: JB Lippincott Co;1162–1196.
4. FIGO cancer committee. Staging announcement. Gynecol Oncol. 1986. 25:383–385.
5. McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996. 334:1–6.
6. Ozols RF. Recurrent ovarian cancer: evidence-based treatment. J Clin Oncol. 2002. 20:1161–1163.
7. Knapp RC, Friedman EA. Aortic lymph node metastases in early ovarian cancer. Am J Obstet Gynecol. 1974. 119:1013–1017.
8. Chen SS. Survival of ovarian carcinoma with or without lymph node metastasis. Gynecol Oncol. 1987. 27:368–372.
9. Spirtos NM, Gross GM, Freddo JL, Ballon SC. Cytoreductive surgery in advanced epithelial cancer of the ovary: the impact of aortic and pelvic lymphadenectomy. Gynecol Oncol. 1995. 56:345–352.
10. The oncology committee of the International Federation of Gynecology and Obstetrics. FIGO news: changes to the 1985 FIGO report on the result of treatment in gynaecological cancer. Int J Gynecol Obstet. 1987. 25:87–88.
11. Hacker NF, Berek JS, Lagasse LD, Nieberg RK, Elashoff RM. Primary cytoreductive surgery for epithelial ovarian cancer. Obstet Gynecol. 1983. 61:413–420.
12. Einhorn N, Nilsson B, Sjöall K. Factors influencing survival in carcinoma of the ovary. Study from a well-defined Swedish population. Cancer. 1985. 55:2019–2025.
13. Hoskins WJ, Bundy BN, Thigpen JT, Omura GA. The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 1992. 47:159–166.
14. Farias-Eisner R, Teng F, Oliveira M, Leuchter R, Karlan B, Lagasse LD, et al. The influence of tumor grade, distribution, and extent of carcinomatosis in minimal residual stage III epithelial ovarian cancer after optimal primary cytoreductive surgery. Gynecol Oncol. 1994. 55:108–110.
15. Kaplan EL, Meier P. Nonparametric extimator from incomplete observations. J Am Stat Assoc. 1958. 53:457–481.
16. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966. 50:163–170.
17. Cox DR. Regression models and life tables. R Stat Soc Bull. 1972. 34:187–220.
18. Meier WJ. Lymphadenectomy in stage I ovarian cancer. Proc Am Soc Clin Oncol. 2000. 19:1541.
19. Benedetti-Panici P, Greggi S, Maneschi F, Scambia G, Amoroso M, Rabitti C, et al. Anatomical and pathological study of retroperitoneal nodes in epithelial ovarian cancer. Gynecol Oncol. 1993. 51:150–154.
20. Petru E, Lahousen M, Tamussino K, Pickel H, Stranzl H, Stettner H, et al. Lymphadenectomy in stage I ovarian cancer. Am J Obstet Gynecol. 1994. 170:656–662.
21. Baiocchi G, Raspagliesi F, Grosso G, Fontanelli R, Cobellis L, di Re E, et al. Early ovarian cancer: Is there a role for systematic pelvis and para-aortic lymphadenectomy? Int J Gynecol Cancer. 1998. 8:103–108.
22. Burghardt E, Girardi F, Lahousen M, Tamussino K, Stettner H. Patterns of pelvic and paraaortic lymph node involvement in ovarian cancer. Gynecol Oncol. 1991. 40:103–106.
23. Lanza A, D'addato F, Valli M, Bussone R, Caldarola B, Re A, et al. Pelvic and para-aortic lymph nodal positivity in the ovarian carcinoma: its prognostic significance. Eur J Gynaecol Oncol. 1988. 9:36–39.
24. Carnino F, Fuda G, Ciccone G, Iskra L, Guercio E, Dadone D, et al. Significance of lymph node sampling in epithelial carcinoma of the ovary. Gynecol Oncol. 1997. 65:467–472.
25. Kanazawa K, Suzuki T, Tokashiki M. The validity and significance of substage IIIC by node involvement in epithelial ovarian cancer: impact of nodal metastasis on patient survival. Gynecol Oncol. 1999. 73:237–241.
26. Cliby WA, Aletti GD, Wilson TO, Podratz KC. Is it justified to classify patients to Stage IIIC epithelial ovarian cancer based on nodal involvement only? Gynecol Oncol. 2006. 103:797–801.
27. Onda T, Yoshikawa H, Yasugi T, Mishima M, Nakagawa S, Yamada M, et al. Patients with ovarian carcinoma upstaged to stage III after systematic lymphadenctomy have similar survival to Stage I/II patients and superior survival to other Stage III patients. Cancer. 1998. 83:1555–1560.
28. Herzog TJ. Assessing the adequacy of surgical staging for ovarian cancer. Gynecol Oncol. 2006. 103:781–782.
29. Ferrandina G, Scambia G, Legge F, Petrillo M, Salutari V. Ovarian cancer patients with "node-positive-only" Stage IIIC disease have a more favorable outcome than Stage IIIA/B. Gynecol Oncol. 2007. 107:154–156.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download