Abstract
Lymphangioleiomyomatosis is a rare disease that is characterized by proliferation of abnormal smooth muscle-like cells, especially that which occurs in the pulmonary parenchyme. It primarily affects women of child-bearing age. The majority of primary lymphangioleiomyomatosis occurs in the lung, but there are a few reports of extrapulmonary cases. We experienced a rare case of lymphangioleiomyomatosis which originated in the pelvic cavity (in the posterior portion of the uterus), and report with brief review of literatures.
Lymphangioleiomyomatosis is a very rare disease which shows typical features of abnormal smooth muscle cell proliferation and which develops in females during the reproductive period.1,2 The majority cases of this disease primarily occur in the lungs, but extrapulmonary regions such as the pelvis and retroperitoneal spaces are occasionally primary sites. Clinical features are the presence of a palpable abdominal mass, abdominal pain, and chylous ascites.3 The etiology and the effective treatment modality are yet unknown, even though many studies regarding lymphangioleiomyomatosis have been reported. We report an experience of a extrapulmonary lymphangiomyomatosis eg, intrapelvic lymphangioleiomyomatosis that developed in the posterior part of the uterus.
A 46-year-old nulligravida presented with complaints of vaginal bleeding for 15 days. Her past history was uneventful except for a left salpingectomy for a benign cyst at age 29. Transabdominal sonography revealed a 5.57×2.91 cm sized hypervascular tumor between the uterus and the right ovary, and two small myomas about 2 cm in size (Fig. 1). Under the impression of ovarian malignancy she had admitted for further evaluation including MRI. Her initial serum CA-125 level was 26.7 U/ml and CA 19-9 level was below 2 U/ml, and other hematologic findings were all within the normal range. Magnetic resonance imaging study of the abdomen-pelvis demonstrated an approximately 4.0×5.0×4.0 cm sized tumor situated at the right posterior of the uterus near to and below the right ovary. This mass showed low signal intensity on T1W1 image and intermediate signal intensity on T2W1 image (Fig. 2). Many congested vessels were observed below the tumor with central liquified or necrotic tissues. Scanty amount of fluid was seen in the cul de sac but no abnormal enlarged lymph nodes. She underwent exploratory laparotomy under general anesthesia. The uterus was normal in size, and a hen-egg sized, irregular margined, easily friable tumor was seen to protrud from the right posterior wall of uterus. Total hysterectomy with right salpingo-oophorectomy, bilateral pelvic lymph node dissection, and bilateral paraaortic lymph node dissection were performed after the frozen biopsy reported that malignancy could not be excluded. The left tube and ovary were abscent and small amount of ascites and very severe adhesion to the bladder and peritoneum were seen.
Gross findings: The uterus measured 10.0×7.0×4.0 cm, six intramural leiomyomatous nodules of yellowish white trabeculated cut surfaces (up to 2.5 cm in greatest dimension) were seen. The endometrium, cervix and attached right adnexa were unremarkable. Several fragments of irregular tan soft masses were present independently.
Microscopic findings: The soft masses consisted of spindle cells arranged in short fascicles around dilated lymphatics or a ramifying network of endothelium-lined spaces. The cells were plump with eosinophilic cytoplasm and nuclei devoid of pleomorphism and mitotic activity. Neither necrosis nor hemorrhage was seen in the mass (Fig. 3). Immunochemical stainings showed that the tumor cells were diffusely positive for smooth muscle actin and strongly multifocally positive for HMB 45 (Fig. 4). The above histologic and immunohistochemical findings were consistent with lymphangioleiomyomatosis. An additional dissection for pelvic lymph node and paraaortic lymph node was performed and revealed nodal metastasis of the two right external iliac, one common iliac and one paraaortic lymph nodes. Tumor cells were also seen in the capsular surfaces and periadnexal soft tissues of the right ovary.
Postoperative condition was fair, and the patient discharged on the 15th postoperative day without complications. After the final diagnosis of lymphangiomyomatosis, chest and brain CT and PET-CT were performed which showed no evidence of metastasis. OPD follow up is ongoing with GnRH-a injection. 6 months has passed with no evidence of recurrence.
Lymphangioleiomyomatosis is a rare disease that is characterized by abnormal proliferation of smooth muscle cells, and develops in the reproductive age female.1,2 Smooth muscle cells are classified in the family of perivascular epithelioid cells (PEC), and examples are renal angioyolipomaa, sugar tumors of the lung and pancreas, clear cell myomelanocytic tumor of the falciform ligament. These so-called "PEComa" all express HMB-45.4 The majority of lymphangiomyomatosis represent cystic lung lesion, lymphatic abnormality and abdominal tumors (ie. angiomyolipoma).1,2,5,6 Pulmonary lymphangiomyomatosis clinically presents with progressive breathlessness or recurrent pneumothorax, chylous pleural effusion, or ascites.6
Otherwise, extrapulmonary lymphangiomyomatosis is very rare and the etiology or the effective treatments are yet unknown. But the clinical features have been described by Matsui K et al.3 and Jaiswal VR et al.7 The clinical features of the extrapulmonary lymphangiomyomatosis are palpable abdominal mass, abdominal pain, and chylous ascites. This tumor is mainly located in the retroperitoneum, pelvic cavity, and the posterior mediastinum along the lymphatic channels. These clinical features are similar to lymphomas or ovarian cancers, so care should be taken when formulating a diagnosis. According to Matsui K et al.3, the diagnosis of pulmonary lymphangioleiomyomatosis is established after that of extrapulmonary lymphangioleiomyomatosis, usually within 2 years. Also, Kim HS et al.8 reported a case of extrapulmonary lymphangioleiomyomatosis in which chylous pleural effusion was also identified two months after the diagnosis. Therefore, extrapulmonary lymphangiomyomatosis should be carefully followed up to discover any lung lesions that develop after the initial diagnosis.
The diagnosis of lymphangiomyomatosis should be considered in a woman of any age who presents with recurrent pneumothorax, chylous pleural effusion and/or ascites, or an unexplained decrease in exercise tolerance. The single most important diagnostic test is a CT scan of the thorax, with high resolution views to facilitate visualization of the cyst. The majority of extrapulmonary lymphangioleiomyomatosis are confirmed after surgery, and in order to differentiate from the other diseases, SMA (smooth muscle α-actin), vimentin, desmin, and HMB-45 (human melanoma black-45) stains should be performed. Especially, HMB-45 is specific for this disease and is essential for correct diagnosis.9,10
The cause of lymphangioleiomyomatosis is not clear yet, but there is a reliable theory. That is, lymphangiomyomatosis occurs in about 30% of woman with the tuberous sclerosis complex (TSC), a genetic disorder of highly variable penetrance associated with seizure, brain tumors and cognitive impairment, and also in woman who do not have TSC. Lymphangioleiomyomatosis is classified by as a tuberous sclerosis associated form or a sporadic form. Both are associated with mutations in the tuberous sclerosis gene, TSC1, TSC2.9-11
Even though many studies have been published to date, the effective treatment mode for lymphangioleiomyomatosis is still obscure. But considering the fact that lymphangioleiomyomatosis usually develops in females of reproductive age,1,2 and that cells of lymphangioleiomyomatosis contain receptors for estrogen and progesterone,12 that pregnancy and estrogen administration aggravates lymphangioleiomyomatosis,13,14 the etiology of this disease is closely related to hormones. The current treatment modality for lymphangioleiomyomatosis is primarily based on the antagonism of estrogen action, and are empiric and unproven. The most commonly employed treatment is IM progesterone which became the standard of care following a dramatic case report in 1987.15 Recently, the use of oral progestins or GnRH-a (Gonadotropin releasing hormone agonists) has also been reported in case studies and small series.12,16,17
Despite a wide variety of treatment modes that have been introduced since the first description of lymphangioleiomyomatosis, patient survival has not improved appreciably. Because of the slow progression of the disease, a report has shown that mortality can within 10 years after diagnosis.18 Although the prognosis of extrapulmonary lymphangioleiomyomatosis is different from that of pulmonary lymphangioleiomyomatosis, because this disease tend to develop pulmonary lymphangioleiomyomatosis after 1-2 years after diagnosis, probably there is no significant difference.
Extrapulmonary lymphangioleiomyomatosis is so rare that there are very few case reports in Korea. We offer in this report information that lymphangioleiomyomatosis which is known as rare a pulmonary disease can develop in the pelvic cavity, and we hope to contribute to the understanding about this disease.
Figures and Tables
Fig. 1
Between the uterus and the right ovary, 5.57×2.91 cm sized solid mass like shadow is seen with high vascularity.
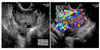
Fig. 2
About 4.0×5.0×4.0 cm sized lobulated mass (☆) is seen in the right posterolateral portion of the uterus. This mass shows low signal intensity on T1W1 (A) and intermediate signal intensity on T2W1 (B). The mass abuts the middle portion of the uterus.
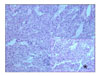
Fig. 3
The tumor is composed of spindle cells, which are arranged in short fascicles around the dilated lymphatic vessels (H&E, ×100). The tumor cells are plump with abundant eosinophilic cytoplasm and nuclei devoid of pleomorphism and arranged around a ramifying network of endothelium-lined spaces (H&E, ×200)(*).
References
1. Johnson SR, Tattersfield AE. Clinical experience of lymphangioleiomyomatosis in the UK. Thorax. 2000. 55:1052–1057.
2. Ryu JH, Moss J, Beck GJ, Lee JC, Brown KK, Chapman JT, et al. The NHLBI Lymphangioleiomyomatosis Registry: Characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006. 173:105–111.
3. Matsui K, Tatsuguchi A, Valencia J, Yu Z, Bechtle J, Beasley MB, et al. Extrapulmonary lymphangioleiomyomatosis (LAM): Clinicopathologic features in 22 cases. Hum Pathol. 2000. 31:1242–1248.
4. Zamboni G, Pea M, Martignoni G, Zancanaro C, Faccioli G, Gilioli E, et al. Clear cell "sugar" tumor of the pancreas. A novel member of the family of lesions characterized by the presence of perivascular epithelioid cells. Am J Surg Pathol. 1996. 20:722–730.
5. Kitaichi M, Nishimura K, Itoh H, Izumi T. Pulmonary lymphangiomyomatosis: A report of 46 patients including a clinicopathologic study of prognostic factors. Am J Respir Crit Care Med. 1995. 151:527–533.
6. Chu SC, Horiba K, Usuki J, Avila NA, Chen CC, Travis WD, et al. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest. 1999. 115:1041–1052.
7. Jaiwal VR, Baird J, Fleming J, Miller DS, Sharma S, Molberg K. Localized retroperitoneal lymphangioleiomyomatosis mimicking malignancy. Arch Pathol Lab Med. 2003. 127:879–882.
8. Kim HS, Park MI, Suh KS. Lymphangiomyomatosis arising in the pelvic cavity: A case report. J Korean Med Sci. 2005. 20:904–907.
9. Taveira-DaSilva AM, Steagall WK, Moss J. Lymphangioleiomyomatosis. Cancer Control. 2006. 13:276–285.
10. McCormack FX. Lymphangioleiomyomatosis: A clinical update. Chest. 2008. 133:507–516.
11. Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2000. 97:6085–6090.
12. Ohori NP, Yousem SA, Sonmez-Alpan E, Colby TV. Estrogen and progesterone receptors in lymphangioleiomyomatosis, epithelioid hemangioendothelioma, and sclerosing hemangioma of the lung. Am J Clin Pathol. 1991. 96:529–535.
13. Brunelli A, Catalini G, Fianchini A. Pregnancy exacerbating unsuspected mediastinal lymphangioleioyomatosis and chylothorax. Int J Gynaecol Obstet. 1996. 52:289–290.
14. Yano S. Exacerbation of pulmonary lymphangioleiomyomatosis by exogenous oestrogen used for infertility treatment. Thorax. 2002. 57:1085–1086.
15. Sieker HO, McCarty KS Jr. Lymphangiomyomatosis: A respiratory illness with an endocrinologic therapy. Trans Am Clin Climatol Assoc. 1987. 99:57–67.
16. Harari S, Cassandro R, Chiodini J, Taveira-DaSilva AM, Moss J. Effect of a gonadotrophin-releasing hormone analogue on lung function in lymphangioleiomyomatosis. Chest. 2008. 133:448–454.
17. Rossi GA, Balbi B, Oddera S, Lantero S, Ravazzoni C. Response to treatment with an analog of luteinizing-hormone-releasing hormone in a patient with pulmonary lymphangioleiomyomatosis. Am Rev Respir Dis. 1991. 143:174–176.
18. Sullivan EJ. Lymphangioleiomyomatosis: A review. Chest. 1998. 114:1689–1703.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download