Abstract
Objective
To compare plasma protein expression between patients with squamous cell carcinoma (SCC) of the cervix and normal controls.
Methods
Plasma samples from patients with benign gynecological disease (normal cervix, n=6) and cervical cancer (SCC, n=6) were subjected to plasma proteomic analysis using two dimensional gel electrophoresis (2-DE) and matrix-assisted laser desorption/ionization mass spectroscopy (MALDI-MS). Western blotting and immunoturbidimetric assay were performed to validate the results of 2-DE.
Results
Eight proteins showed differential expression between controls and SCC patients; six (ceruloplasmin, complement C3, afamin precursor, alpha-1-B-glycoprotein, transferrin, alpha-fibrinogen precursor) were up-regulated, while two (chain A, crystal structure of antithrombin and apolipoprotein A-IV precursor) were down-regulated in the plasma of SCC patients. Western blotting analysis revealed significant elevation of ceruloplasmin, complement C3, afamin, and alpha-1-B-glycoprotein in the plasma of SCC patients in comparison to controls. Immunoturbidimetric assay of a larger group confirmed the results of 2-DE and Western blotting, and showed that ceruloplasmin and complement C3 were significantly elevated in the plasma of SCC patients in comparison with controls and patients with carcinoma in situ (CIS) of the uterine cervix.
Uterine cervical cancer is the second most common type of cancer and is the third most frequent cause of cancer death in women worldwide, with 493,243 new cases and 273,505 deaths in 2002.1 Cervical cancer is more common in developing countries, where 83% of cervical cancer occurs and where it accounts for 15% of female cancers.1 In contrast, cervical cancer constitutes only 3.6% of new cancers in developed countries.1 Recently, there have been substantial decreases in both the incidence and mortality of cervical cancer in developed countries where effective screening programs are in place, while the incidence and mortality have increased in developing countries.1,2
The diagnosis of cervical cancer is based on the results of cytological and histological examinations. Although the Pap smear for cytological examination is effective for screening cervical cancer, the evaluation of the Pap smear relies on subjective diagnostic skills and experience, which may result in sampling errors or errors in the interpretation of abnormal cells. In addition, the infrastructure for Pap smear screening is not well established in most developing countries.3-5 Although colposcopy-guided biopsy and cone biopsy for histological examination can be used to diagnose cervical cancer, they are expensive procedures that require specific skills to perform.6 Therefore, the discovery of biomarkers for simple, inexpensive, and widespread testing in developing countries is needed for the screening, early detection, and post-treatment surveillance of cervical cancer.
Human blood plasma and serum are a useful source of disease biomarkers that likely contain information related to cancer development and progression.7-9 Therefore, plasma and serum are the preferred type of specimen for screening, early detection, and post-treatment surveillance of cancer, because plasma and serum samples are the most accessible human body fluid from which a sample can be acquired and are readily available using less invasive, inexpensive methods. Also, use of plasma and serum specimen for biomarker of cervical cancer may supplement problems of the Pap smear which has a lower compliance for screening and follow up in developing countries. Between plasma and serum, plasma tends to be preferable for many laboratory investigators, as the components of plasma better reflect the pathological status of a patient than serum10,11
To identify new cancer biomarkers, proteomic analysis with two dimensional gel electrophoresis (2-DE) and matrix assisted laser desorption/ionization mass spectrometry (MALDI-MS) have been used to examine the differential expression of protein in serum or plasma specimens from different tumors, such as nasopharyngeal carcinoma, gastric cancer, and hepatocellular carcinoma.12-14 The advantage of 2-DE combined with MALDI-MS is that it can detect differences in the expression levels of various proteins between healthy normal controls and cancer patients both qualitatively and quantitatively.15
This study was performed to evaluate the expression of plasma proteins using 2-DE combined with MALDI-MS in patients with squamous cell carcinoma (SCC) of the uterine cervix and patients with benign gynecologic disease as normal controls.
Plasma samples were obtained from six patients with primary cervical cancer treated at Busan Paik Hospital, Inje University, Busan, Korea. The diagnosis was confirmed as SCC histologically in all cases. None of the patients received any treatment before the primary treatment described here. The stage of cervical cancer was established according to the International Federation of Gynecology and Obstetrics (FIGO) criteria: 2, 2, 1, and 1 cancers were classified as FIGO stage Ib, IIa, IIb, and IIIb, respectively. As controls, plasma samples were obtained from six patients with normal cervices who were treated for benign gynecological diseases, including uterine adenomyosis (2 cases), leiomyoma (1 cases), and benign ovarian tumor (3 cases). All patients provided informed consent before collection of the plasma samples. The collection and use of the samples was approved by the Institutional Review Board of Busan Paik Hospital (IRB number 06-14).
To improve the 2-DE of human plasma samples, the albumin and immunoglobulin G in the collected plasma samples were depleted using an Albumin and IgG Removal Kit (GE Healthcare, Buckinghamshire, UK) in accordance with the manufacturer's instructions.
The albumin and immunoglobulin G-depleted plasma samples were mixed with 4 volumes of pre-cooled acetone (-20℃, 15 min) and incubated for 2 h at -20℃ for protein precipitation. The protein pellets were obtained after high speed centrifugation (13,000 × g for 10 min, at 4℃), and then air-dried for 5-10 min at room temperature. The pellets were stored at -80℃ until analysis.
The plasma protein pellets were dissolved in lysis buffer (7 M urea, 2 M thiourea, 4% CHAPS, 40 mM Tris base, 1% DTT, 0.5% IPG buffer, 0.5% Triton X-114, and protease inhibitor cocktail) and kept at room temperature for 1 h. The protein content was assayed using a 2-D Quant kit (GE Healthcare, Buckinghamshire, UK) with serum BSA (0-50 µg/µL) as a standard.
Dried 24-cm (pH 3-10NL) immobilized pH gradient (IPG) strips were rehydrated overnight in a rehydration tray with 450 µL of Destreak (tm) rehydration solution (GE Healthcare, Buckinghamshire, UK) containing 2% IPG buffer (v/v). Following rehydration, aliquots of 100 µg of the soluble plasma proteins in 100 µL of sample solution were loaded using the cup -loading method. Isoelectric focusing (IEF) was carried out at 60,000 V/h at 20℃ as follows: 500 V for 1 h, 1,000 V for 1 h, and finally 8,000 V incremented to 60,000 V/h. The IPG strips were placed in 5 ml of equilibration solution (50 mM Tris-HCl, pH 8.8, containing 6 M urea, 30% glycerol, 2% SDS, and bromophenol blue) containing 1% DTT (v/v) during the first equilibration step and 2.5% iodoacetamide (v/v) during the second equilibration step (15 min per equilibration step). The 2-D separation was performed using the Ettan DALT twelve system (GE Healthcare, Buckinghamshire, UK). The IPG strips were loaded onto a 12.5% gel for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), running buffer (25 mM Tris, 192 mM glycine, 3.5 mM SDS, pH 8.3) was added, and a constant current (5 W/gel) was applied for 6 h. The gels were then stained with silver nitrate. The stained gels were scanned on a PowerLook 1100 flatbed scanner (UMAX, Fremont, CA, USA), and the images were analyzed using commercial software (Image Master 2D Platinum; GE Healthcare, Buckinghamshire, UK).
For MS fingerprinting, the stained portions of the 2-D gels were excised and then digested with trypsin as described by Rosenfeld et al.16 Isolated protein spots were destained with 100 mM sodium thiosulfate and 30 mM potassium ferricyanide. After washing with 50% acetonitrile (AcN), the gel fragments were dried in a vacuum centrifuge. The dried gel fragments were rehydrated in 20 mL of 25 mM NH4HCO3 containing 0.5 mg of sequencing-grade trypsin (Promega, Madison, WI, USA), and incubated overnight at 37℃. The remaining peptides were extracted twice with 30 mL of a 50 mM NH4HCO3:AcN (1:1) mixture. The extracts were evaporated in a vacuum centrifuge for further drying. Aliquots of the peptide-containing samples were applied to a target disk and left to evaporate. Spectra were obtained using a Voyager DE PRO MALDI-MS (Applied Biosystems, Foster City, CA, USA). Protein databases were searched with MASCOT using monoisotope peaks. A mass tolerance within 50 ppm was allowed initially, after which recalibration was performed using the list of proteins obtained within a tolerance of 20 ppm.
Aliquots of 20 µg of plasma protein were separated by 10% SDS-PAGE. The proteins were transferred onto polyvinylidene fluoride (PVDF) membranes, which were blocked overnight in TBS (20 mM Tris and 150 mM NaCl, pH 8.0) containing 5% non-fat dry milk, and then probed with antibodies for ceruloplasmin, complement C3, afamin, and alpha-1-B glycoprotein at a dilution of 1 mg/mL for 1 h at room temperature. The membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody at a dilution of 1:2000 for 1 h at room temperature. Immunoreactivity was visualized with an ECL Western blotting detection kit (Amersham Biosciences, Piscataway, NJ, USA) using an LAS 3000 image detector (Fuji Film, Miyagi, Japan).
A new set of plasma samples for validation was collected that included 24 normal controls treated for benign gynecological diseases with normal cervices, 26 patients with carcinoma in situ (CIS) of the uterine cervix, and 27 patients with SCC of the cervix at FIGO stages I to IV; the recurrent cervical cancer was in 14, 8, 2, 2, and 3 cases, respectively. These plasma samples for validating the immunoturbidimetric assay were obtained from Busan Paik Hospital, Inje University, Busan, South Korea. All subjects provided informed consent before collection of plasma samples. The collection and use of the samples were approved by the Institutional Review Board of Busan Paik Hospital (IRB number 06-14). The immunoturbidimetric assays for ceruloplasmin and complement C3 were performed using a Cobas Integra device (Roche, Basel, Switzerland) at Seoul Medical Science Institute (SCL) with ceruloplasmin regent (Roche, Basel, Switzerland) or complement C3 reagent, respectively. No assays were performed for afamin or alpha-1-B glycoprotein because commercial assay kits are not available.
Statistical analysis was performed using MedCalc® version 9.5.2 (MedCalc Software, Mariakerke, Belgium). Student's t-test and analysis of variance (ANOVA) were used to analyze differences among the normal controls, CIS, and SCC cervical cancer patients. Differences were considered statistically significant at p<0.05.
Proteome analysis was performed using 2-DE and silver staining of plasma samples from normal controls and patients with SCC of the cervix. Using an automated spot-counting algorithm provided by Image Master 2D Platinum, more than 400 protein spots were detected in each gel image. The expression patterns of the plasma proteins are shown in Fig. 1 (A, normal control; B, cervical cancer). Computer-assisted comparative analysis of the respective spot patterns matched over 30 spots and selected 15 spots that changed significantly, which showed a constant expression rate that was over 120%, or decreased to less than 80% of that in the controls (Fig. 2). In SCC of the cervix, there were 11 up-regulated spots and four down-regulated spots.
Fifteen differentially expressed spots in the 2-DE gels were isolated and subjected to MALDI-MS. The peptide mass peaks were compared to those in the NCBI database (Fig. 3). The identified protein MS data descriptions are listed in Table 1. Only eight proteins were identified from the 15 differentially expressed spots because spots from the same area were identified as one protein. Of these eight proteins, the six that were up-regulated in the plasma of SCC compared to normal controls were ceruloplasmin, complement C3, afamin precursor, alpha-1-B glycoprotein, transferrin, and alpha-fibrinogen precursor. The remaining two proteins that were down-regulated in SCC were chain A, and crystal structure of antithrombin and apolipoprotein A-IV precursor.
Western blotting analysis was performed to confirm the 2-DE results for the up-regulated proteins. Of the six up-regulated proteins, antibodies against four proteins were available commercially: ceruloplasmin, complement C3, afamin, and alpha-1-B glycoprotein. Consistent with the 2-DE data, Western blotting analyses of ceruloplasmin, complement C3, afamin, and alpha-1-B glycoprotein showed significant elevation of these proteins in the plasma of patients with SCC of the cervix in comparison with the normal controls (Fig. 4).
We performed an immunoturbidimetric assay with a new set of plasma samples to confirm the 2-DE and Western blotting results for the up-regulated proteins for further validation. Of the up-regulated proteins, tests for ceruloplasmin and complement C3 were available commercially. Consistent with the 2-DE and Western blotting data, the immunoturbidimetric assays of ceruloplasmin [cut-off value 22, sensitivity 74.1% (95% CI, 53.7-88.8); specificity 62.5% (95% CI, 40.6-81.2)] and complement C3 [cut-off value 112, sensitivity 51.9% (95% CI, 32.0-71.3)], specificity 83.3% (95% CI, 62.6-95.2)] showed significant elevation of these proteins in the plasma of patients with SCC of the cervix compared with normal controls and patients with CIS of the cervix (p<0.05; Fig. 5, Table 2). However, there were no associations between expression for the two proteins and clinicopathological prognostic parameters: stage, depth of stromal invasion, lymphovascular space invasion, parametrium invasion, and lymph node metastasis (p>0.05, data not shown).
In this study, we showed significant elevation of ceruloplasmin, complement C3, afamin, and alpha-1-B glycoprotein in the plasma of patients with SCC of the cervix compared to normal controls. Although we did not show associations between expression for ceruloplasmin and complement C3 and clinicopathological prognostic parameters, we demonstrated that ceruloplasmin and complement C3 were significantly elevated in the plasma of patients with SCC of the cervix compared to normal controls and CIS of the cervix using larger numbers of samples. We consider that a further, large-scale study is necessary to establish associations between expression for the two proteins and clinicopathological prognostic parameters.
Ceruloplasmin is a copper-carrying protein that acts as the major ferroxidase and stimulates iron uptake into cells, which is necessary for cell proliferation.17 Ceruloplasmin is also a hypoxia-induced gene, linking the hypoxia and copper pathways of angiogenesis.17,18 Angiogenesis is the initial step in progressive tumor development and metastasis.19 The first stage in tumor angiogenesis is the activation of endothelial cells.19 Copper stimulates the proliferation and migration of endothelial cells, and activates several proangiogenic factors including vascular endothelial growth factor, basic fibroblast growth factor, tumor necrosis factor alpha, and interleukin.19 In the absence of copper, apoceruloplasmin is secreted, but is degraded rapidly.17 Increased expression of ceruloplasmin has been reported in the serum of several cancer patients.12,20-22
Complement components play a central role as mediators of inflammation and regulate of the immune response.23 Complement C3, the third component of complement, is not only the most abundant complement protein in human serum, but also plays a central role in the complement system.24 Complement C3 is a multipotent protein that participates in different events involved in the immune response, as including complement activation, antigen presentation, cell-cell interactions, and cell proliferation.25 Complement activation with the subsequent deposition of complement components on tumor tissues has been demonstrated in cancer patients.26-30 Hanas et al. reported that complement C3 was elevated in the serum of patients with pancreas cancer.31 Lee et al. found that complement C3a was elevated in the serum of patients with hepatitis C-related hepatocellular carcinoma.32 In addition, Bjorgem et al. demonstrated that complement C3a was elevated in the ascitic fluids of ovarian cancer patients.33 Therefore, complement C3 may be a marker related to carcinogenesis.
Afamin, or alpha-albumin, is a new protein and is the fourth member of the albumin family, which comprises albumin, alpha-fetoprotein, and vitamin D binding protein.34,35 Afamin is present in the plasma/serum, cerebrospinal fluid, and follicular fluid.35,36 Afamin is considered a vitamin E binding protein, and vitamin E has been shown to play a crucial role in protection against oxidative damage and disease.35,36 Wu et al.37 reported that afamin was significantly down-regulated in hepatoma tissue. Jackson et al.38 showed that afamin was decreased in the serum of ovarian cancer patients. In contrast, we found that afamin was significantly elevated in the plasma of patients with SCC of the cervix. Therefore, the expression and role of afamin in carcinogenesis should be investigated further.
Alpha-1-B glycoprotein is a plasma protein of unknown function.39 Given its internal duplication and its sequence homology to immunoglobulin-like proteins, alpha-1-B glycoprotein appears to have evolved from an ancestral gene similar to that of the immunoglobulin supergene family.39 Alpha-1-B glycoprotein is present in normal adult plasma at an average concentration of 22 mg/dl.39 However, no biological function has yet been proposed for this protein. There has been one previous study regarding alpha-1-B glycoprotein in the blood of cancer patients. Abdul-Rahman et al. reported that alpha-1-B glycoprotein level was elevated in the serum of patients with endometrial and cervical cancer.40 As the function of alpha-1-Bglycoprotein is unknown, the biological function of alpha-1-B glycoprotein and its association with cancer should be examined in future studies.
In conclusion, we identified ceruloplasmin, complement C3, afamin, and alpha-1-B glycoprotein in plasma as potential biomarkers related to SCC of the cervix using 2-DE combined with MALDI-MS. A further validation study of afamin and alpha-1-B glycoprotein, as biomarkers of SCC of the cervix using larger numbers of samples is needed. In addition, further functional studies of these proteins will provide more information on their roles in the development and progression of cervical cancer.
Figures and Tables
Fig. 1
2-DE analysis of plasma proteins in normal controls (A) and patients with squamous cell carcinoma of the cervix (B). Fifteen spots that showed significant changes were selected for MALDI-MS analysis.
Fig. 2
(A) Enlarged 2-DE images showing up- and down-regulation of expression of plasma proteins in squamous cell carcinoma of the cervix compared to normal controls. (B) Histogram of different plasma protein levels in normal and cervical cancer.
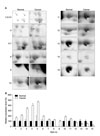
Fig. 3
MALDI-MS multipotent spectra of afamin isolated from a 2-DE plasma protein gel. Representative spectra for 17 peaks are shown. The 13 matched peptides are shown in bold on the peaks.

Fig. 4
Plasma proteins obtained from normal controls and patients with squamous cell carcinoma of the cervix were subjected to Western blotting analysis with antibodies to ceruloplasmin, afamin, complement C3, and alpha-1-B glycoprotein (A1BG). (A) Equal amounts of protein (20 µg) were analyzed by SDS-PAGE. (B) Histogram of the four different proteins in normal controls and patients with squamous cell carcinoma of the cervix (p<0.05).

Fig. 5
Plasma proteins obtained from 24 normal controls, 26 patients with CIS of the cervix, and 27 patients with squamous cell carcinoma of the cervix were subjected to immunoturbidimetric assays with ceruloplasmin and complement C3 antibodies. These proteins were significantly elevated in the plasma of patients with squamous cell carcinoma of the cervix compared with normal controls and patients with CIS of the cervix (p<0.05).

References
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005. 55:74–108.
2. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. 2008. Fort Washington: National Comprehensive Cancer Network.
3. Liao SY, Stanbridge EJ. Expression of MN/CA9 protein in Papanicolaou smears containing atypical glandular cells of undetermined significance is a diagnostic biomarker of cervical dysplasia and neoplasia. Cancer. 2000. 88:1108–1121.
4. Kurman RJ, Henson DE, Herbst AL, Noller KL, Schiffman MH. Interim guidelines for management of abnormal cervical cytology: The 1992 National Cancer Institute Workshop. JAMA. 1994. 271:1866–1869.
5. Koss LG. Cervical (Pap) smear: New directions. Cancer. 1993. 71:4 Suppl. 1406–1412.
6. Lin YW, Lai HC, Lin CY, Chiou JY, Shui HA, Chang CC, et al. Plasma proteomic profiling for detecting and differentiating in situ and invasive carcinomas of the uterine cervix. Int J Gynecol Cancer. 2006. 16:1216–1224.
7. Shen Y, Kim J, Strittmatter EF, Jacobs JM, Camp DG 2nd, Fang R, et al. Characterization of the human blood plasma proteome. Proteomics. 2005. 5:4034–4045.
8. Shoshan SH, Admon A. Proteomics in clinical laboratory diagnosis. Adv Clin Chem. 2005. 39:159–184.
9. Joo WA, Lee DY, Kim CW. Development of an effective sample preparation method for the proteome analysis of body fluids using 2-D gel electrophoresis. Biosci Biotechnol Biochem. 2003. 67:1574–1577.
10. Anderson NL, Polanski M, Pieper R, Gatlin T, Tirumalai RS, Conrads TP, et al. The human plasma proteome: A non-redundant list developed by combination of four separate sources. Mol Cell Proteomics. 2004. 3:311–326.
11. Jacobs JM, Adkins JN, Qian WJ, Liu T, Shen Y, Camp DG 2nd, et al. Utilizing human blood plasma for proteomic biomarker discovery. J Proteome Res. 2005. 4:1073–1085.
12. Doustjalali SR, Yusof R, Govindasamy GK, Bustam AZ, Pillay B, Hashim OH. Patients with nasopharyngeal carcinoma demonstrate enhanced serum and tissue ceruloplasmin expression. J Med Invest. 2006. 53:20–28.
13. Liu W, Liu B, Xin L, Zhang Y, Chen X, Zhu Z, et al. Down-regulated expression of complement factor I: a potential suppressive protein for gastric cancer identified by serum proteome analysis. Clin Chim Acta. 2007. 377:119–126.
14. Feng JT, Liu YK, Song HY, Dai Z, Qin LX, Almofti MR, et al. Heat-shock protein 27: A potential biomarker for hepatocellular carcinoma identified by serum proteome analysis. Proteomics. 2005. 5:4581–4588.
15. Wilkins MR, Pasquali C, Appel RD, Ou K, Golaz O, Sanchez JC. From proteins to proteomes: Large-scale protein identification by two-dimensional electrophoresis and amino acid analysis. Biotechnology. 1996. 14:61–65.
16. Rosenfeld J, Capdevielle J, Guillemot JC, Ferrara P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal Biochem. 1992. 203:173–179.
17. Lowndes SA, Harris AL. The role of copper in tumour angiogenesis. J Mammary Gland Biol Neoplasia. 2005. 10:299–310.
18. Martin F, Linden T, Katschinski DM, Oehme F, Flamme I, Mukhopadhyay CK, et al. Copper-dependent activation of hypoxia-inducible factor (HIF)-1: Implications for ceruloplasmin regulation. Blood. 2005. 105:4613–4619.
19. Nasulewicz A, Mazur A, Opolski A. Role of copper in tumour angiogenesis: Clinical implications. J Trace Elem Med Biol. 2004. 18:1–8.
20. Pejovic M, Djordjevic V, Ignjatovic I, Stamenic T, Stefanovic V. Serum levels of some acute phase proteins in kidney and urinary tract urothelial cancers. Int Urol Nephrol. 1997. 29:427–432.
21. Agroyannis B, Dalamangas A, Dardouphas K, Fortoynas C, Saloum G, Stringou E, et al. Serum transferrin and ceruloplasmin in patients with cancer of the gastrointestinal and other systems. Anticancer Res. 1994. 14:2201–2203.
22. Ros-Bullón MR, Sánchez-Pedreño P, Martínez-Liarte JH. Serum ceruloplasmin in melanoma patients. Anticancer Res. 2001. 21:629–632.
23. Jurianz K, Ziegler S, Garcia-Schüler H, Kraus S, Bohana-Kashtan O, Fishelson Z, et al. Complement resistance of tumor cells: Basal and induced mechanisms. Mol Immunol. 1999. 36:929–939.
24. Sahu A, Lambris JD. Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol Rev. 2001. 180:35–48.
25. Frade R. Structure and functions of proteases which cleave human C3 and are expressed on normal or tumor human cells: Some are involved in tumorigenic and metastatic properties of human melanoma cells. Immunopharmacology. 1999. 42:39–45.
26. Lucas SD, Karlsson-Parra A, Nilsson B, Grimelius L, Akerstrom G, Rastad J, et al. Tumor-specific deposition of immunoglobulin G and complement in papillary thyroid carcinoma. Hum Pathol. 1996. 27:1329–1335.
27. Niculescu F, Rus HG, Retegan M, Vlaicu R. Persistent complement activation on tumor cells in breast cancer. Am J Pathol. 1992. 140:1039–1043.
28. Yamakawa M, Yamada K, Tsuge T, Ohrui H, Ogata T, Dobashi M, et al. Protection of thyroid cancer cells by complement-regulatory factors. Cancer. 1994. 73:2808–2817.
29. Bernet-Camard MF, Coconnier MH, Hudault S, Servin AL. Differential expression of complement proteins and regulatory decay accelerating factor in relation to differentiation of cultured human colon adenocarcinoma cell lines. Gut. 1996. 38:248–253.
30. Niehans GA, Cherwitz DL, Staley NA, Knapp DJ, Dalmasso AP. Human carcinomas variably express the complement inhibitory proteins CD46 (membrane cofactor protein), CD55 (decay-accelerating factor), and CD59 (protectin). Am J Pathol. 1996. 149:129–142.
31. Hanas JS, Hocker JR, Cheung JY, Larabee JL, Lerner MR, Lightfoot SA, et al. Biomarker identification in human pancreatic cancer sera. Pancreas. 2008. 36:61–69.
32. Lee IN, Chen CH, Sheu JC, Lee HS, Huang GT, Chen DS, et al. Identification of complement C3a as a candidate biomarker in human chronic hepatitis C and HCV-related hepatocellular carcinoma using a proteomics approach. Proteomics. 2006. 6:2865–2873.
33. Bjørge L, Hakulinen J, Vintermyr OK, Jarva H, Jensen TS, Iversen OE, et al. Ascitic complement system in ovarian cancer. Br J Cancer. 2005. 92:895–905.
34. Bélanger L, Roy S, Allard D. New albumin gene 3' adjacent to the alpha 1-fetoprotein locus. J Biol Chem. 1994. 269:5481–5484.
35. Lichenstein HS, Lyons DE, Wurfel MM, Johnson DA, McGinley MD, Leidli JC, et al. Afamin is a new member of the albumin, alpha-fetoprotein, and vitamin D-binding protein gene family. J Biol Chem. 1994. 269:18149–18154.
36. Jerkovic L, Voegele AF, Chwatal S, Kronenberg F, Radcliffe CM, Wormald MR, et al. Afamin is a novel human vitamin E-binding glycoprotein characterization and in vitro expression. J Proteome Res. 2005. 4:889–899.
37. Wu GX, Lin YM, Zhou TH, Gao H, Pei G. Significant down-regulation of alpha-albumin in human hepatoma and its implication. Cancer Lett. 2000. 160:229–236.
38. Jackson D, Craven RA, Hutson RC, Graze I, Lueth P, Tonge RP, et al. Proteomic profiling identifies afamin as a potential biomarker for ovarian cancer. Clin Cancer Res. 2007. 13:7370–7379.
39. Ishioka N, Takahashi N, Putnam FW. Amino acid sequence of human plasma alpha 1B-glycoprotein: Homology to the immunoglobulin supergene family. Proc Natl Acad Sci U S A. 1986. 83:2363–2367.
40. Abdul-Rahman PS, Lim BK, Hashim OH. Expression of high-abundance proteins in sera of patients with endometrial and cervical cancers: analysis using 2-DE with silver staining and lectin detection methods. Electrophoresis. 2007. 28:1989–1996.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download