Abstract
Objective
The purposes of this study were to evaluate the expression of p16INK4a (referred as to p16) and Ki-67 in cervical intraepithelial neoplasia (CIN), and the correlation between high-risk human papillomavirus (HPV) infection and the above biomarkers.
Methods
We analyzed 31 patients who were diagnosed with CIN at Kwandong University Myongji Hospital from October 2006 to September 2007. CIN specimens (CIN1, 12; CIN2, 6; CIN3, 13) were obtained by colposcopy-directed biopsy (CDB) or loop electrical excision procedure (LEEP). The expressions of p16 and Ki-67 were evaluated by immunohistochemical methods with antibodies to p16 and Ki67. The immunohistochemical staining results were classified into four grades: 0, 1, 2 and 3. HPV genotyping or Hybrid Capture-II test was used to detect high-risk HPV.
Results
The expression of p16 (p<0.001) and Ki-67 (p=0.003) were positively associated with CIN grade. p16 expressions increased significantly with high-risk HPV infection (p=0.014), especially HPV type 16 and 58. Ki-67 expression was not related with high-risk HPV. There was positive correlation between the expression of the p16 and Ki-67 (p=0.007).
Cervical cancer is still one of the common cancers in Korea. Cervical cancer is known to develop from precancerous disease, cervical intraepithelial neoplasia (CIN). CIN takes 5 to 15 years to progress to invasive cancer. By extensive epidemiologic and molecular biologic studies, the human papillomavirus (HPV) infection is known to be the most important etiology of cervical cancer.1 HPV is a double-stranded DNA virus and over 120 types of HPV have been identified till now. HPV is classified into high-risk and low-risk HPV. The persistent infection of high-risk HPV is associated with development of cervical cancer.2-4
HPV is known to induce cervical cancer through uncontrolled G1-S transition. The E6 and E7 proteins of high-risk HPV inhibit the p53 and pRb proteins which are cell cycle regulatory proteins controlling G1-S transition.5 The p16INK4a (p16) is a protein which belongs to the inhibitors of cyclin-dependent kinase (CDK) 4 family (INK4a family). By interacting with CDK4 and CDK6, p16 inhibits the formation of cyclin D/CDK4 and 6 complex, which is a proliferation-stimulating protein. The p16 also functions as a cyclin-dependent kinase inhibitor (CDKI) by inhibiting the CDK-induced phosphorylation of pRb.6,7 The phosphorylation of pRb induces the release of a transcription factor E2F from the bound form of E2F and pRb. The release of E2F results in G1-S transition.8 Like the p16 protein, HPV infection induces the release of E2F through the binding of E7 to pRb. The released E2F stimulates the expression of genes which are involved in G1-S transition.9 The inactivation of pRb by E7 causes the p16 overexpression because p16 is regulated by negative feedback of pRb.9-12 Ki-67 is a well-known cell proliferation marker and which may be used for grading CIN.13-15
To evaluate the clinical values of p16 and Ki-67 expressions, we examined the p16 and Ki-67 expressions in CIN and investigated the associations of high-risk HPV infection with the p16 and Ki-67 expressions.
Thirty-one patients who underwent a colposcopy-directed biopsy or loop electrosurgical excision procedure and were diagnosed as having CIN at the Myongji Hospital between October 2006 and September 2007 were included in this study. Normal cervical tissues which were located next to a CIN lesion on a slide were used as controls.
Tests for high-risk HPV infection were performed at the time of the biopsy. Oligonucleotide microarray DNA chip (MyGene Inc., Seoul, Korea) or HPV hybrid capture II® kit (Digene/Abbott, Clopper Road, Gaithersburg, Maryland, USA) were used to detect the high-risk HPV. HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68 were considered as the high-risk HPV, and HPV 6, 11, 34, 40, 42, 43, 44, 70 were regarded as the low-risk HPV.
Formalin-fixed, paraffin-embedded tissue blocks were sliced in thickness of 3 um and the tissue sections were mounted on silanized slides. Immunohistochemical staining was performed through the indirect biotin streptoavidin method using the iVIEW™ DAB Detection Kit (Ventana Medical Systems, Tucson, AZ, USA). The sections were deparaffinized in xylene and were sequentially washed twice in 100% alcohol and in 95%, 90%, 80%, and 70% alcohol for two minutes. To increase the antigen detection, the slides were immersed in a citrate acid solution and were heated for 20 minutes in a microwave. The slides were washed with APK Wash Solution (Ventana Medical Systems, Tucson, AZ, USA) and were stained using the automatic immunohistochemical staining equipment, Ventana NexES IHC (Ventana Medical Systems, Tucson, AZ, USA). The p16 and Ki-67 staining was performed with 1:25 diluted Monoclonal Mouse Antibody p16INK4a protein (Diagnostic Bio-System, USA) and 1:50 diluted Monoclonal Mouse Antibody (DAKOCytomation, Denmark), respectively.
After the slides were incubated with antibodies for 32 minutes, the slides were exposed to Diaminobenzidine (DAB) for 4 minutes and were counterstained with Mayer's Hematoxylene for 4 minutes. DAB and Mayer's Hematoxylene which were included in iVIEW™ DAB Detection Kit (Ventana Medical Systems, Tucson, AZ, USA) were used for staining. All staining procedures were performed at 37℃. Stained slides were dried and were covered with glass cover slides. For a negative control, non-immune mouse serum IgG was used instead of primary antibodies.
All slides were examined by two independent reviewers. Irrespective of cytoplasmic staining, the cell whose nucleus was stained with anti-p16 antibody was regarded as p16 positive. The percentage of p16 positive cells was used to determine the grade of p16 expression. Grade 0 was given when the percentage of positive cells was below 1%. Grade 1+ and 2+ were assigned when the clustered positive cells were present and the percentage of positive cells was 1-5% and 5-25%, respectively. Grade 3+ was graded when there were diffuse positive cells and the percentage of positive cells was greater than 25%. To determine the grade of Ki-67 expression, nucleuses of 200 epithelial cells located across the whole epithelial layer were examined in a high-power field (×600). Ki-67 index was defined as the percentage of Ki-67 positive cells. Grade 1+, 2+, and 3+ was given when the Ki-67 index was below 5%, 5-30%, and greater than 30%, respectively.
The association of CIN grade with high-risk HPV infection and p16, Ki-67 expressions were evaluated with the Fisher's exact test, Mann-Whitney test, Kruskall-Wallis test, and Pearson's correlation test using SPSS 13.0 (Chicago, IL, USA). p values smaller than 0.05 were regarded to be statistically significant.
Twelve patients with CIN 1 (38.7%), six patients with CIN 2 (19.4%), and 13 patients with CIN 3 (41.9%) were included in this study. The results of the pathologic examination and HPV test, the grade of p16 and Ki-67 expression are summarized in Table 1 and Fig. 1. The p16 staining was performed in 31 patients but Ki-67 staining and HPV tests were performed in 30 patients. The p16 expression was not observed in 10 of 12 patients with CIN 1 (83.3%), but strong p16 expression was detected in all patients with CIN 3 (13/13). Ki-67 expression was detected in all patients. Seven of 12 patients with CIN 1 showed weak Ki-67 expression, but 6 of 12 patients with CIN 3 had strong Ki-67 expression (Table 2). As the CIN grade was higher, stronger p16 and Ki-67 expressions were observed (p16, p<0.001; Ki-67, p=0.003; Fig. 2). In addition, the expression level of p16 positively correlated with that of Ki-67 (p=0.007).
HPV test was performed in 30 patients. Two patients underwent the HPV hybrid capture-II test and 28 patients received the HPV DNA genotyping. Among the 30 patients who underwent the HPV test, 21 patients demonstrated high-risk HPV infection. Sixteen of 19 patients with CIN 2 or 3 (84.2%) and five of 11 patients with CIN 1 (45.5%) were positive for high-risk HPV (p=0.035; Table 3). HPV 16 was the most common type of HPV detected in 31 patients and HPV 58, 52 were the second and third most common type of HPV. HPV 16 and 58 were detected only in high-grade CIN. In three patients, both high-risk and low-risk HPV were identified. However, there were no patients who were infected with only low-risk HPV (Table 1). Out of nine patients with negative HPV test, the p16 expression was not observed in six patients (66.7%) and strong p16 expression was detected in two patients (22.2%). However, strong p16 expression was observed in all patients with HPV 16 or 58 infections (Table 4). Among 21 patients with high-risk HPV infection, p16 expression was not detected in three patients (14.3%) and strong expression was identified in 14 patients (66.7%). High-risk HPV infection was associated with p16 expression (p=0.014; Table 4). The Ki-67 expression was detected in all patients. Ki-67 expression was not associated with HPV infection or high-risk HPV infection (Table 4). When we examined the expression levels of p16 and Ki-67 according to the HPV type, the expression level of p16 was higher in patients with high-risk HPV infection than in patients without high-risk HPV infection (Fig. 3). However, HPV 52 infection was not associated with the expression levels of p16 or Ki-67. In addition, the expression level of Ki-67 was not associated with HPV infection (Fig. 3).
HPV infection is known as the most important cause of cervical cancer. The inhibition of cell cycle regulatory proteins by E6 and E7 is known to initiate the carcinogenesis process. The p16 is a cell cycle regulatory protein which is the main target of HPV, and Ki-67 is a cell proliferation marker. We examined the association of the high-risk HPV infection with the expression levels of p16 and Ki-67 in patients with CIN. In early reports on cell cycle regulatory proteins, the association of the CIN grade with the expression level of p16 was unclear. However, recent studies reported that the p16 and Ki-67 expressions were higher in high-grade CIN than in low-grade CIN (Table 5).12,16-21 The results of the recent studies are concordant with that of the current study. The mechanism of p16 overexpression is still unclear. Some researchers hypothesized that the p16 overexpression may be due to the removal of p16 inhibition by pRb, which is degraded by E7 through a ubiquitin-dependent proteinase system.22-24 Several studies have reported that the p16 expression increased in patients with a high-risk HPV infection.17,25 These findings indirectly supported the hypothesis. In the current study, p16 expression increased in patients with high-risk HPV infection. In the current study, as the CIN grade was higher, the p16 and Ki-67 expressions became stronger. The Ki-67 expression was not associated with high-risk HPV infection. These findings suggest that p16 may be involved in the HPV-induced carcinogenesis. To increase the reproducibility of diagnosis, Ki-67 may be employed as an objective marker because the expression levels of Ki-67 linearly increase as the CIN grade is higher. Although Ki-67 may be used as a marker for cell proliferation, Ki-67 is thought not to play a role in carcinogenesis of cervical cancer.
The association of HPV type with expression levels of p16 is still controversial. Previous studies reported that HPV 16 was associated with expression levels of p16.18,20 In the current study, the expression level of p16 was increased in patients infected with HPV 16, the strongest oncogenic virus. HPV 58 and 52, whose prevalence are known to be higher in Korea than in other countries, are the most common HPV types except HPV 16.23 Like HPV 16, HPV 58 is associated with the expression levels of p16. Therefore, HPV 58 is thought to be related with the development of cervical cancer in Korean women. There were only few studies which have investigated the association of HPV 58 with p16 overexpression. The p16 is thought to be related with the carcinogenesis process induced by HPV 58. Therefore, further studies on the expression of cell cycle regulatory proteins in patients infected with HPV 58 are necessary. For a HPV vaccine to be effective in Korea, the HPV vaccine should target HPV 52 and 58, in addition to the four types of HPV (HPV 16, 18, 6, 11) which are already included in the currently available HPV vaccine.
In low grade CIN, HPV test was negative in 54.5% of patients. The hybrid capture-II test may be negative in CIN 1 because CIN 1 develops from the low-risk HPV. However, HPV DNA genotyping which detects all types of HPV was performed for most patients in the current study. Therefore, the high negative rate of HPV test is thought to be due to the insensitivity of HPV test. In addition, there were no patients who were infected with only low-risk HPV. The p16 overexpression is rare in patients infected with a low-risk HPV because E7 of low-risk HPV has lower affinity to pRb than that of high-risk HPV.27
In conclusion, the expression level of p16 and Ki-67 increased as the CIN grade was higher. The p16 overexpression was associated with a high-risk HPV infection. Especially, the p16 overexpression was associated with HPV 58 infection. Cell cycle regulatory proteins related with p16 are thought to play a role in carcinogenesis process induced by HPV 58.
Figures and Tables
Fig. 1
Representative figures of immunohistochemical staining for p16INK4a (A-D) and Ki-67 (E-G) in dysplastic cervical tissues. (A) 0 expression (CIN 2), (B) 1+ expression (CIN 2), (C) 2+ expression (CIN 2), (D) 3+ expression (CIN 3), (E) 1+ expression (CIN 3), (F) 2+ expression (CIN 3), (G) 3+ expression (CIN 3) (magnification, ×400).
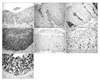
Fig. 2
p16 and Ki-67 expression status according to the grade of CIN. The expression of p16 (p<0.001) and Ki-67 (p=0.003) were positively associated with CIN grade.

Fig. 3
p16 and Ki-67 expression status according to the status of HPV infection. p16 expressions increased significantly with high-risk HPV infection (p=0.014), especially HPV type 16 and 58. Ki-67 expression was not related with high-risk HPV.

References
1. zur Hausen H. Human papillomaviruses and their possible role in squamous cell carcinomas. Curr Top Microbiol Immunol. 1977. 78:1–30.
2. Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: A worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995. 87:796–802.
3. Lorincz AT, Reid R, Jenson AB, Greenberg MD, Lancaster W, Kurman RJ. Human papillomavirus infection of the cervix: Relative risk associations of 15 common anogenital types. Obstet Gynecol. 1992. 79:328–337.
4. Pfister H. Human papillomaviruses and genital cancer. Adv Cancer Res. 1987. 48:113–147.
5. Slebos RJ, Lee MH, Plunkett BS, Kessis TD, Williams BO, Jacks T, et al. p53-dependent G1 arrest involves pRB-related proteins and is disrupted by the human papillomavirus 16 E7 oncoprotein. Proc Natl Acad Sci U S A. 1994. 91:5320–5324.
6. Nam EJ, Kim YT. Alteration of cell-cycle regulation in epithelial ovarian cancer. Int J Gynecol Cancer. 2008. Feb. 19. [Epub ahead of print].
7. Nam EJ, Kim HY, Kim SW, Yoon BS, Kim JH, Kim YT, et al. Relationship between p16INK4a, pRb and high risk HPV infection and recurrence. Korean J Obstet Gynecol. 2006. 49:1437–1445.
8. Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991. 65:1053–1061.
9. Dyson N, Howley PM, Munger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989. 243:934–937.
10. Kamb A, Gruis NA, Weaver-Feldhaus J, Liu Q, Harshman K, Tavtigian SV, et al. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994. 264:436–440.
11. Sherr CJ. Cancer cell cycles. Science. 1996. 274:1672–1677.
12. Nam EJ, Kim JW, Kim SW, Kim YT, Kim JH, Yoon BS, et al. The expressions of the Rb pathway in cervical intraepithelial neoplasia; predictive and prognostic significance. Gynecol Oncol. 2007. 104:207–211.
13. al-Saleh W, Delvenne P, Greimers R, Fridman V, Doyen J, Boniver J. Assessment of Ki-67 antigen immunostaining in squamous intraepithelial lesions of the uterine cervix. Correlation with the histologic grade and human papillomavirus type. Am J Clin Pathol. 1995. 104:154–160.
14. Pirog EC, Baergen RN, Soslow RA, Tam D, DeMattia AE, Chen YT, et al. Diagnostic accuracy of cervical low-grade squamous intraepithelial lesions is improved with MIB-1 immunostaining. Am J Surg Pathol. 2002. 26:70–75.
15. Isacson C, Kessis TD, Hedrick L, Cho KR. Both cell proliferation and apoptosis increase with lesion grade in cervical neoplasia but do not correlate with human papillomavirus type. Cancer Res. 1996. 56:669–674.
16. Keating JT, Cviko A, Riethdorf S, Riethdorf L, Quade BJ, Sun D, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001. 25:884–891.
17. Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, et al. Overexpression of p16INK4a as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001. 92:276–284.
18. Agoff SN, Lin P, Morihara J, Mao C, Kiviat NB, Koutsky LA. p16INK4a expression correlates with degree of cervical neoplasia: A comparison with Ki-67 expression and detection of high-risk HPV types. Mod Pathol. 2003. 16:665–673.
19. Wang SS, Trunk M, Schiffman M, Herrero R, Sherman ME, Burk RD, et al. Validation of p16INK4a as a marker of oncogenic human papillomavirus infection in cervical biopsies from a population-based cohort in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2004. 13:1355–1360.
20. Murphy N, Ring M, Heffron CC, King B, Killalea AG, Hughes C, et al. p16INK4a, CDC6, and MCM5: Predictive biomarkers in cervical preinvasive neoplasia and cervical cancer. J Clin Pathol. 2005. 58:525–534.
21. Lorenzato M, Caudroy S, Bronner C, Evrard G, Simon M, Durlach A, et al. Cell cycle and/or proliferation markers: What is the best method to discriminate cervical high-grade lesions. Hum Pathol. 2005. 36:1101–1107.
22. Sakaguchi M, Fujii Y, Hirabayashi H, Yoon HE, Komoto Y, Oue T, et al. Inversely correlated expression of p16 and Rb protein in non-small cell lung cancers: An immunohistochemical study. Int J Cancer. 1996. 65:442–445.
23. Hofmann F, Martelli F, Livingston DM, Wang Z. The retinoblastoma gene product protects E2F-1 from degradation by the ubiquitin-proteasome pathway. Genes Dev. 1996. 10:2949–2959.
24. Hateboer G, Kerkhoven RM, Shvarts A, Bernards R, Beijersbergen RL. Degradation of E2F by the ubiquitin-proteasome pathway: Regulation by retinoblastoma family proteins and adenovirus transforming proteins. Genes Dev. 1996. 10:2960–2970.
25. Song SH, Park HM, Eom DW, Lee JK, Lee NW, Kim AR, et al. The expression of p16INK4a and Ki-67 in relation to high-risk human papilloma viral load and residual disease after conization with positive margins. Int J Gynecol Cancer. 2007. 17:858–867.
26. Hwang HS, Park M, Lee SY, Kwon KH, Pang MG. Distribution and prevalence of human papillomavirus genotypes in routine pap smear of 2,470 Korean women determined by DNA chip. Cancer Epidemiol Biomarkers Prev. 2004. 13:2153–2156.
27. Gage JR, Meyers C, Wettstein FO. The E7 proteins of the non-oncogenic human papillomavirus type 6b (HPV-6b) and of the oncogenic HPV-16 differ in retinoblastoma protein binding and other properties. J Virol. 1990. 64:723–730.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download