Abstract
Pelvic actinomycosis is an uncommon disease in humans. It has nonspecific and variable clinical features, and thus it is difficult to diagnose. Moreover, appropriate management is delayed or overlooked because it can sometimes simulate advanced ovarian cancer. We report a case of pelvic actinomycosis which manifested with hydronephrosis and bowel stricture, lymph node enlargement and increased level of tumor marker caused by a large pelvic mass. Since this case showed clinical findings mimicking an advanced ovarian carcinoma, it was surgically diagnosed as actinomycosis after neoadjuvant chemotherapy.
Actinomycosis is a chronic suppurative granulomatous disease mostly caused by Actinomyces israelii (A. israelii), a gram-positive anaerobic bacilli.1 Pelvic actinomycosis, accompanied by intrauterine devices (IUD) in the majority of cases, is rare and accounts for about 3% of all actinomycosis.2,3 Though it shows clinical features like tuboovarian abscess, it may simulate pelvic malignancies or retroperitoneal tumors. It is sometimes difficult to diagnose pelvic actinomycosis before surgery.
We present a case of pelvic actinomycosis simulating an advanced ovarian cancer where an increase in CA125 level and direct invasion into the urinary bladder and colon was present, which led to hydronephrosis and colonal stricture confirmed by computed tomography (CT). The CA 125 level returned to normal and her symptoms, such as pain and constipation, improved after 3 cycles of neoadjuvant chemotherapy.
A 42-year-old married woman, gravida 4 para 2, was referred to the Center for Uterine Cancer of National Cancer Center for pelvic discomfort, pelvic mass and constipation. She complained of pelvic discomfort for 6 months. She had no specific family history of medical diseases except that her mother had gastric cancer. An IUD had been inserted 8 years earlier and removed 4 months ago because of pelvic discomfort.
On physical examination, her body temperature was 36.5℃, her blood pressure 123/82 mmHg and her pulse rate 99/min and regular. Vaginal examination revealed a firm and slightly tender mass measuring approximately the uterine size of a 14-week pregnancy, which was attached to the pelvis. The uterus and adnexa could not be differentiated due to the congregated solid pelvic mass.
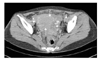
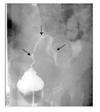
Laboratory tests showed no specific findings except for anemia (hemoglobin, 8.1 g/dL) and 1+ proteinuria. The CA 125 level measured by radioimmunoassay increased to 139 U/ml (normal range, 0-35 U/ml). Papanicolaou-stained cytologic smear was negative. CT revealed a pelvic mass extending into the uterus, adnexa and rectosigmoid colon, which caused left hydronephrosis by compression on the ureter (Fig. 1). This tumor seemed to invade the root of the inferior mesenteric artery, part of the small intestine as well as the rectosigmoid colon and bladder wall. Colon study with barium contrast demonstrated extrinsic compression of the descending and sigmoid colons (Fig. 2). Gastroendoscopy and colonoscopy exhibited normal findings except for extrinsic compression on the colon.
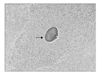
All of these findings suggested the presence of advanced ovarian cancer. Ascites tapping or percutaneous needle biopsy was not performed because there was no ascites and because the small bowel enclosed the mass. Since it was thought that primary debulking operation would be difficult due to invasion of the root of the inferior mesenteric artery and muscles of the bladder, the patient was treated with neoadjuvant chemotherapy using 3 cycles of paclitaxel-cisplatin regimen. The CA 125 level decreased to 34 U/ml and her symptom, such as pain and constipation, improved after chemotherapy. Based on these findings, we performed exploratory laparotomy for debulking. There was a mass involving the uterus, adnexa and distal colon with severe adhesion between the mass and pelvic organs including the uterus, small and large bowels, and bladder. It compressed the left ureter and caused ureteral dilatation. En-bloc excision of the mass was performed with total hysterectomy, bilateral salpingo-oophorectomy, low anterior resection with reanastomsis and appendectomy. Since the frozen section revealed actinomycosis, the patient underwent prophylactic ileostomy and insertion of a double J catheter into the left ureter. She received antibiotic therapy. Histology confirmed a definitive diagnosis of bilateral salpingo-ovarian actinomycosis which showed sulfur granule and formed an abscess with invasion into the uterus, rectosigmoid colon and appendix (Fig. 3). She received intravenous penicillin G (15×106 IU) for 4 weeks and oral penicillin for additional 6 months. After completion of antibiotic therapy, she underwent repair of ileostomy. She had neither gynecologic symptoms nor urinary/bowel complaints during a follow-up period of 1 year.
Actinomycosis is usually caused by A. israelii but it can be also caused by A. bovis, A. ericksonii, A naeslundii, A. viscousus, or A. odonlyticus. Actinomyces species are gram-positive, anaerobic or microaerophilic, non-spore-forming bacilli which produce sulfur granule in the tissue. These bacteria are part of endogenous microflora in the oro-pharyngeal cavity and become pathogenic when they enter the peritoneal cavity.2-4 Pelvic actinomycosis usually occurs following bowel perforation, especially perforation of the appendix due to appendicitis, or following ascending infections through the IUD. It can also be caused by direct spread or hematologic dissemination in systemic disease. These lead to tissue granulation, dense fibrosis and abscess in the pelvis. These lesions can produce a hard mass in the pelvis and it may compress the ureter or intestines.5 Until now, more than fifteen cases of Actinomycosis have been reported showing bowel stricture or hydronephrosis in Korea.6-8 Hydronephrosis with ureteral obstruction is related to the presence of an IUD in most cases and can be relieved by antibiotic medication and transient insertion of a ureteral stent. Our case also showed the resolution of left ureteral dilatation and hydronephrosis following insertion of a temporary double J catheter and administration of antibiotics as in previously reported cases.9-13 Patients with colorectal stricture were also successfully treated with antibiotic therapy, although some underwent intestinal resection, as in our case.11-15 In severe cases, patients may receive emergency en-bloc resection of the right colon, upper rectum and sigmoid colon, a portion of the abdominal wall, and left ovary because of bowel obstruction.16
In addition, many authors have demonstrated other clinical features simulating ovarian cancer including large mass with ascites, lymph node enlargement, and increased level of tumor marker. However there is no report showing all features consisting of bowel stricture, hydronephrosis, ascites, lymph node enlargement and increased level of tumor marker. In current case, the patient represented these clinical features except ascites.
Patients complain of abdominal pain (85%), weight loss (44%) and foul-odorous vaginal discharge (24%). Abdominal tenderness and fever are noted in 60% of patients on physical examination. Thus, the clinical findings of pelvic actinomycosis are similar to those of tubo-ovarian or pelvic abscesses. Laboratory tests reveal anemia, leukocytosis and an elevated erythrocyte sedimentation rate in many cases. Since it shows nonspecific findings, it is difficult to distinguish pelvic actinomycosis from other pelvic inflammatory diseases. Moreover, pelvic actinomycosis can simulate pelvic malignancies in many cases because of its non-specific clinical features, presence of a solid invasive mass and lack of marked leukocytosis.5,17 There have been several reports of pelvic actinomycosis mimicking malignancies. Hoffman et al. reported 2 cases of actinomycotic pelvic inflammatory disease simulating an advanced ovarian carcinoma or advanced cervical carcinoma.18 Koshiyama et al. presented pelvic actinomycosis which was treated with neoadjuvant chemotherapy, carboplatin, doxorubicin and cyclophosphamide, because it was misdiagnosed as an advanced ovarian cancer.19 Since cultures of Actinomyces species show very low yield, it is difficult to distinguish a solid mass of pelvic actinomycosis from a malignant tumor before surgery.
Our patient did not present with fever, leukocytosis or elevated ESR although anemia was present. When the patient visited our clinic, the IUD had been removed. In our case, there were an elevation of the CA 125 level, and the CT revealed an aggregated mass involving the root of the inferior mesenteric artery, part of the small intestine as well as the uterus, adnexa, rectosigmoid colon and bladder wall. The CA 125 level returned to normal and her symptoms, such as pain and constipation, improved after chemotherapy. Therefore, it seemed that she had an advanced cancer rather than inflammatory disease. Lee et al. reported that CT-guided core needle biopsy can be an alternative diagnostic tool instead of open laparotomy for the diagnosis of pelvic actinomycosis.20 However, we did not perform a biopsy or peritoneal cytology prior to chemotherapy because the small bowel enclosed the mass and also because there was no ascites.
In conclusion, since pelvic actinomycosis not only has nonspecific and varied clinical features, and since it may also simulate pelvic malignancies, its diagnosis is relatively difficult. We report a case of pelvic actinomycosis manifested by hydronephrosis and bowel stricture, lymph node enlargement, and increased level of tumor marker caused by a large pelvic mass.
Figures and Tables
References
1. Berardi RS. Abdominal actinomycosis. Surg Gynecol Obstet. 1979. 149:257–266.
2. Henderson R. Pelvic actinomycosis associated with an intrauterine device. Obstet Gynecol. 1973. 41:726–732.
3. Hamid D, Baldauf JJ, Cuenin C, Ritter J. Treatment strategy for pelvic ctinomycosis: Case report and review of the literature. Eur J Obstet Gynecol Reprod Biol. 2000. 89:197–200.
4. Brown JR. Human actinomycosis: A study of 181 subjects. Hum Pathol. 1973. 4:319–330.
5. Fiorino AS. Intrautrine contraceptive device-associated actonomycotic abscess and actinomycosis detection on cervical smear. Obstet Gynecol. 1996. 87:142–149.
6. Bae JJ, Kim JH, Park YK, Lee DJ, Koh MW, Lee TH, et al. A clinical analysis of pelvic actinomycosis. Korean J Obstet Gynecol. 2007. 50:1132–1140.
7. Cho JH, Lee YJ, Kim DH. A case of pelvic actinomycosis associated with and intrauterine contraceptive device. Korean J Obstet Gynecol. 1986. 29:1048–1052.
8. Hyung WJ, Kim MW, Kim MK, Chang DY, Jeon MK, Lee ES. 4 cases of pelvic actinomycosis associated with intrauterine contraceptive device. Korean J Obstet Gynecol. 2005. 48:509–518.
9. Fulton IC, Paterson WG, Crucioli V. Pelvic actinomycosis causing ureteric obstruction. Case reports. Br J Obstet Gynaecol. 1981. 88:1044–1050.
10. Palmar TE, Venable DD. Retroperitoneal actinomycosis. South Med J. 1986. 79:1301–1303.
11. Haj M, Nasser G, Loberant N, Cohen I, Nesser E, Eitan A. Pelvic actinomycosis presenting as ureteric and rectal stricture. Dig Surg. 2000. 17:414–417.
12. Lininger JR, Frable WJ. Diagnosis of pelvic actinomycosis by fine needle aspiration: A case report. Acta Cytol. 1984. 28:601–604.
13. Nasu K, Matsumoto H, Yoshimatsu J, Miyakawa I. Ureteral and sigmoid obstruction caused by pelvic actinomycosis in an intrauterine contraceptive device user. Gynecol Obstet Invest. 2002. 54:228–231.
14. Spickett GP, Kipping RA. Pelvic actinomycosis presenting with rectal stricture. J R Soc Med. 1985. 78:674–675.
15. Ratliff DA, Carr N, Cochrane JP. Rectal stricture due to actinomycosis. Br J Surg. 1986. 73:589–590.
16. Yeguez JF, Martinez SA, Sands LF, Hellinger MD. Pelvic actinomycosis presenting as malignant large bowel obstruction: A case report and a review of the literature. Am Surg. 2000. 66:85–90.
17. Weese WC, Smith IM. A study of 57 cases of actinomycosis over a 36-year period. A diagnostic 'failure' with good prognosis after treatment. Arch Intern Med. 1975. 135:1562–1568.
18. Hoffman MS, Roberts WS, Solomon P, Gunasekarin S, Cavanagh D. Advanced actinomycotic pelvic inflammatory disease simulating gynecologic malignancy; A report of two cases. J Reprod Med. 1991. 36:543–545.
19. Koshiyama M, Yoshida M, Fujii H, Nanno H, Hayashi M, Tauchi K, et al. Ovarian actinomycosis complicated by diabetes mellitus simulating an advanced ovarian carcinoma. Eur J Obstet Gynecol Reprod Biol. 1999. 87:95–99.
20. Lee YC, Min D, Holcomb K, Buhl A, DiMaio T, Abulafia O. Computed tomography guided core needle biopsy diagnosis of pelvic actinomycosis. Gynecol Oncol. 2000. 79:318–323.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download