Abstract
Endometrial stromal sarcoma (ESS) is a relatively rare uterine sarcoma, especially extrauterine ESS. Furthermore, retroperitoneal ESS are extremely rare. Up to now, there are only four cases of primary retroperitoneal ESS reported in the literature. We report one case of primary retroperitoneal undifferentiated endometrial stromal sarcoma after concurrent chemoradiation therapy for cervical cancer with a brief review of the literature.
Endometrial stromal sarcomas (ESS) are rare uterine malignancies, accounting for less than 1% of all uterine cancers and approximately 7-15% of all uterine sarcomas.1 Extrauterine ESS occasionally develops in the ovary or at extraovarian sites, such as the fallopian tube, vagina, pelvic cavity, abdominal cavity, and retroperitoneum. Because of the rarity of extrauterine ESS, very little is known about its pathogenesis.2-4 This neoplasm may develop from preexisting endometriosis or after radiation treatment, but there is no direct evidence for this.2 Only four cases of primary retroperitoneal ESS have been reported in the literature to date.2,3,5,6
We present the fourth case of a primary retroperitoneal undifferentiated endometrial sarcoma without associated endometriosis that developed four years after concurrent chemoradiation therapy for cervical cancer.
A 76-year-old-woman, gravida 10, para 9, presented with a palpable mass in the left lower abdomen. The medical history included stage IIb non-keratinizing large cell squamous cell carcinoma of the cervix diagnosed four years before presentation. At that time, her serum SCC Ag level was 5.0 ng/mL. She underwent concurrent chemoradiation therapy with 4,500 cGy external radiotherapy, 3,200 cGy intracavitary radiotherapy and six cycles of cisplatin 40 mg/m2/week chemotherapy. Four months later, a follow up pelvic MRI showed nearly total regression of the previously noted malignancy of the cervix. The serum SCC Ag level and cervical Pap smear were normal on follow up. The patient returned for follow up every 3 months for the first 2 years after treatment and then every 6 months. There was no sign of recurrent cervical cancer during the routine follow-up for four years. However, after four years of follow up, the patient suddenly complained of left pelvic pain. Physical examination revealed a normal cervix and parametrium. However, a very large, hard and fixed mass in the left lower abdomen was detected. The cervical Pap smear was normal and the serum SCC Ag level was within normal range. The pelvic MRI showed a 7.5×6.2×5.8 cm heterogeneously enhancing mass encasing the left external iliac vessels, but the uterus, cervix and both ovaries were without abnormality (Fig. 1). Because there was no evidence of recurrence and the mass was bulky, we performed an exploratory laparotomy. An 8 cm firm mass involving the left pelvic wall was identified along the left external iliac vessels. Grossly, the uterus and both adnexae were normal. However, although debulking was carried out, we could not totally resect the mass. Microscopically, the tumor consisted of sheets of round to spindle cells with moderate nuclear pleomorphism (Fig. 2A). The tumor cells had increased mitotic activity (up to 52 per 10 high power fields) (Fig. 2B). In addition, focal areas of necrosis were noted. Despite complete sampling of the tumor, no other components including endometriosis were identified. An immunohistochemical analysis was performed. The tumor cells were strongly positive for vimentin and CD 10, but negative for α-smooth muscle actin, desmin, cytokeratin, CD 34, C-kit, S100 protein, estrogen receptor, and progesterone receptors (Fig. 2C, D). Based on these findings, an undifferentiated ESS was diagnosed. An endometrial biopsy could not be performed due to severe cervical stenosis from the effects of the previous radiation treatments. The postoperative recovery was uneventful and palliative radiation therapy was provided.
ESS is an uncommon neoplasm and its occurrence outside of the uterus is extremely rare in the absence of metastasis or extension of a primary uterine neoplasm. Extrauterine sites of primary ESS include the ovary, Fallopian tube, pelvic cavity, abdominal cavity (subcolonic, mesenteric), and retroperitoneum.2-6 Mostly reported extrauterine sites were intraperitoneal, only four prior cases have been reported in the literature with primarily retroperitoneal ESS (Table 1).
The etiology of ESS is unknown. However, there have been some reports suggesting that ESS is associated with endometriosis.2-4 However, three of the previously reported cases of primary retroperitoneal ESS, and including our case, were not associated with endometriosis. Another possible etiology may be a de novo tumor, derived from submesothelial pluripotential mullerian cells. The pluripotential mullerian epithelium is considered to be widely distributed in the abdominal and pelvic cavities.7 In addition, the carcinogenic effect of radiation therapy has been discussed extensively. Pelvic malignancies commonly reported following radiotherapy for cervical carcinoma include cancers of the uterus, ovaries, bladder, and rectum. Sites within the irradiated field have been implicated in sarcomas of the pelvic girdle, carcinoma of the vagina and carcinoma of the vulva. Carcinoma and sarcoma of the uterus have been considered as possible postirradiation neoplasms. Latency periods of five to 20 years are most often reported. Epidemiological studies have reported no increased incidence of endometrial carcinoma following radiation therapy, whereas sarcoma of the uterus has been shown to have a slightly increased incidence. Most of the postirradiation sarcomas have been mixed mesodermal tumors carcinosarcomas with mixed mesodermal tumors predominanting.8 Our patient was diagnosed with an undifferentiated endometrial sarcoma that may have been associated with the effects of radiation.
Undifferentiated endometrial sarcomas (UES) were previously designated as high-grade endometrial stromal sarcomas. By definition, the proliferation of endometrial stromal cells is associated with an undifferentiated state and is characterized by a population of blunt spindle to oblong cells with little cytoplasm, and relatively small uniform dense nuclei without significant atypia.5 When an ESS is identified in the retroperitoneum, the differential diagnosis should include all of the monomorphous spindled neoplasias that originate from the soft tissue, particularly hemangiopericytoma, neural tumors and smooth muscle tumors.2 Immunohistochemical staining may be useful in the differential diagnosis.9 For our case CD 10 and vimentin were positive whereas actin, desmin, CK, CD34, CD68, c-Kit, S-100, ER, and PR were negative. The microscopic features of the tumor were typical for an undifferentiated sarcoma, and the immunohistochemical findings were consistent with an endometrial stromal sarcoma. In addition, there was no uterine lesion. These findings support the diagnosis of primary extrauterine undifferentiated endometrial sarcoma.
There are no standard guidelines for the management of patients with an extrauterine undifferentiated endometrial stromal sarcoma. We could not totally resect the retroperitoneal tumor therefore, palliative radiation therapy was performed for local treatment. Because of the advanced age and poor performance status of the patient, chemotherapy was not performed.
In summary, this case illustrates the findings of a primary undifferentiated endometrial stromal sarcoma in the retropetrioneum four years after concurrent chemoradiation therapy for cervical cancer.
Figures and Tables
Fig. 1
Pelvic MRI showing a normal uterus (A), and about a 7.5×6.2×5.8 cm retroperitoneal irregular shaped heterogenous enhancing mass that encased the left external iliac vessels (B, C, D). (A) Sagittal T2 weighted image, (B) Axial T1 weighted image, (C) Axial T2 weighted image, (D) Axial gadolinium enhanced T1 weighted image. The arrow points to the left external iliac vessel.
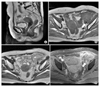
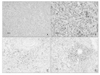
Fig. 2
Histological and immunohistochemical findings. Low magnification shows solid proliferation of oval to spindlecells (A, hematoxylin-eosin, ×40). The tumor cells show nuclear pleomorphism and increased mitotic activity (B, hematoxylin-eosin, ×200). Immunohistochemically, the tumor cells were immunoreactive to vimentin (C, ×200) and CD 10 (D, ×200).
References
1. Leath CA 3rd, Huh WK, Hyde J Jr, Cohn DE, Resnick KE, Taylor NP, et al. A multi-institutional review of outcomes of endometrial stromal sarcoma. Gynecol Oncol. 2007. 105:630–634.
2. Chang KL, Crabtree GS, Lim-Tan SK, Kempson RL, Hendrickson MR. Primary extrauterine endometrial stromal neoplasms: A clinicopathologic study of 20 cases and a review of the literature. Int J Gynecol Pathol. 1993. 12:282–296.
3. Ulbright TM, Kraus FT. Endometrial stomal tumors of extra-uterine tissue. Am J Clin Pathol. 1981. 76:371–377.
4. Young RH, Prat J, Scully RE. Endometrioid stromal sarcomas of the ovary. A clinicopathologic analysis of 23 cases. Cancer. 1984. 53:1143–1155.
5. Morrison C, Ramirez NC, Chan JK, Wakely P Jr. Endometrial stromal sarcoma of the retroperitoneum. Ann Diagn Pathol. 2002. 6:312–318.
6. Kim SW, Choi SH, Lee SK, Lee JJ, Kim MJ, Kim TY, et al. A case of post-total hysterectomy with bilat. salpingooophorectomy retroperitoneal endometrial stromal sarcoma. Korean J Obstet Gynecol. 2006. 49:1803–1807.
7. Lauchlan SC. The secondary Mullerian system. Obstet Gynecol Surv. 1972. 27:133–146.
8. Hoffman M, Roberts WS, Cavanagh D. Second pelvic malignancies following radiation therapy for cervical cancer. Obstet Gynecol Surv. 1985. 40:611–617.
9. Baker P, Oliva E. Endometrial stromal tumours of the uterus: A practical approach using conventional morphology and ancillary techniques. J Clin Pathol. 2007. 60:235–243.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download