Abstract
Psychrobacter sanguinis
has been described as a Gram-negative, aerobic coccobacilli originally isolated from environments and seaweed samples. To date, 6 cases of P. sanguinis infection have been reported. A 53-year-old male was admitted with a generalized tonic seizure lasting for 1 minute with loss of consciousness and a mild fever of 37.8 o C. A Gram stain revealed Gram-negative, small, and coccobacilli-shaped bacteria on blood culture. Automated microbiology analyzer identification using the BD BACTEC FX (BD Diagnostics, Germany) and VITEK2 (bioMérieux, France) systems indicated the presence of Methylobacterium spp., Aeromonas salmonicida, and the Moraxella group with low discrimination. The GenBank Basic Local Alignment Search Tool and an Ez-Taxon database search revealed that the 16S rRNA gene sequence of the isolate showed 99.30% and 99.88% homology to 859 base-pairs of the corresponding sequences of P. sanguinis, respectively (GenBank accession numbers JX501674.1 and HM212667.1). To the best of our knowledge, this is the first human case of P. sanguinis bacteremia in Korea. It is notable that we identified a case based on blood specimens that previously had been misidentified by a commercially automated identification analyzer. We utilized 16S rRNA gene sequencing as a secondary method for correctly identifying this microorganism.
References
1. Bowman JP. The genus Psychrobacter. In: The Prokaryotes. New York: Springer;2006. p. 920–30.
2. Kämpfer P, Albrecht A, Buczolits S, Busse HJ. Psychrobacter faecalis sp. nov., a new species from a bioaerosol originating from pigeon faeces. Syst Appl Microbiol. 2002; 25:31–6.

3. Bozal N, Montes MJ, Tudela E, Guinea J. Characterization of several Psychrobacter strains isolated from Antarctic environments and description of Psychrobacter luti sp. nov. and Psychrobacter fozii sp. nov. Int J Syst Evol Microbiol. 2003; 53:1093–100.

4. Vela AI, Collins MD, Latre MV, Mateos A, Moreno MA, Hutson R, et al. Psychrobacter pulmonis sp. nov., isolated from the lungs of lambs. Int J Syst Evol Microbiol. 2003; 53:415–9.

5. Gini GA. Ocular infection caused by Psychrobacter immobilis acquired in the hospital. J Clin Microbiol. 1990; 28:400–1.

6. Lloyd-Puryear M, Wallace D, Baldwin T, Hollis DG. Meningitis caused by Psychrobacter immobilis in an infant. J Clin Microbiol. 1991; 29:2041–2.

7. Lozano F, Florez C, Recio FJ, Gamboa F, Gómez-Mateas JM, Martín E. Fatal Psychrobacter immobilis infection in a patient with AIDS. AIDS. 1994; 8:1189–90.

8. Leung WK, Chow VC, Chan MC, Ling JM, Sung JJ. Psychrobacter bacteraemia in a cirrhotic patient after the consumption of raw geoduck clam. J Infect. 2006; 52:e169–71.

9. Parving HH, Oxenbøll B, Svendsen PA, Christiansen JS, Andersen AR. Early detection of patients at risk of developing diabetic nephropathy. A longitudinal study of urinary albumin excretion. Acta Endocrinol (Copenh). 1982; 100:550–5.

10. Hudson MJ, Hollis DG, Weaver RE, Galvis CG. Relationship of CDC group EO-2 and Psychrobacter immobilis. J Clin Microbiol. 1987; 25:1907–10.

11. Wirth SE, Ayala-del-Río HL, Cole JA, Kohlerschmidt DJ, Musser KA, Sepúlveda-Torres Ldel C, et al. Psychrobacter sanguinis sp. nov., recovered from four clinical specimens over a 4-year period. Int J Syst Evol Microbiol. 2012; 62:49–54.

12. Le Guern R, Wallet F, Vega E, Courcol RJ, Loïez C. Psychrobacter sanguinis: an unusual bacterium for nosocomial meningitis. J Clin Microbiol. 2014; 52:3475–7.

13. Ortiz-Alcántara JM, Segura-Candelas JM, Garcés-Ayala F, Gonzalez-Durán E, Rodríguez-Castillo A, Alcántara-Pérez P, et al. Fatal Psychrobacter sp. infection in a pediatric patient with meningitis identified by metagenomic next-generation sequencing in cerebrospinal fluid. Arch Microbiol. 2016; 198:129–35.

14. CLSI. Interpretive criteria for identification of bacteria and fungi by DNA target sequencing; approved guidline. CLSI document MM18-A. Wayne, PA: Clinical and Laboratory Standards Institute;2008.
15. Rossau R, Van Landschoot A, Gillis M, De Ley J. Taxonomy of Moraxellaceae fam. nov., a new bacterial family to accommodate the genera Moraxella, Acinetobacter, and Psychrobacter and related organisms. Int J Syst Bacteriol. 1991; 41:310–9.

16. Pettersson B, Kodjo A, Ronaghi M, Uhlén M, Tønjum T. Phylogeny of the family Moraxellaceae by 16S rDNA sequence analysis, with special emphasis on differentiation of Moraxella species. Int J Syst Bacteriol. 1998; 48:75–89.

17. Petti CA, Polage CR, Schreckenberger P. The role of 16S rRNA gene sequencing in identification of microorganisms misidentified by conventional methods. J Clin Microbiol. 2005; 43:6123–5.

18. Woo PC, Lau SK, Teng JL, Tse H, Yuen KY. Then and now: use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin Microbiol Infect. 2008; 14:908–34.

19. Lin YC, Tu CY, Chen W, Tsai YL, Chen HJ, Hsu WH, et al. An urgent problem of aerobic gram-negative pathogen infection in complicated parapneumonic effusions or empyemas. Intern Med. 2007; 46:1173–8.

20. Kihla AJ, Ngunde PJ, Evelyn MS, Gerard N, Ndip RN. Risk factors for wound infection in health care facilities in Buea, Cameroon: aerobic bacterial pathogens and antibiogram of isolates. Pan Afr Med J. 2014; 18:6.

21. Pasala S, Barr T, Messaoudi I. Impact of alcohol abuse on the adaptive immune system. Alcohol Res. 2015; 37:185–97.
22. Zhang P, Bagby GJ, Happel KI, Raasch CE, Nelson S. Alcohol abuse, immunosuppression, and pulmonary infection. Curr Drug Abuse Rev. 2008; 1:56–67.
Fig. 1.
Graph showing change in body temperature, white blood cell count (WBC), absolute neutrophil count (ANC) and C-reactive protein (CRP) levels during admission days.
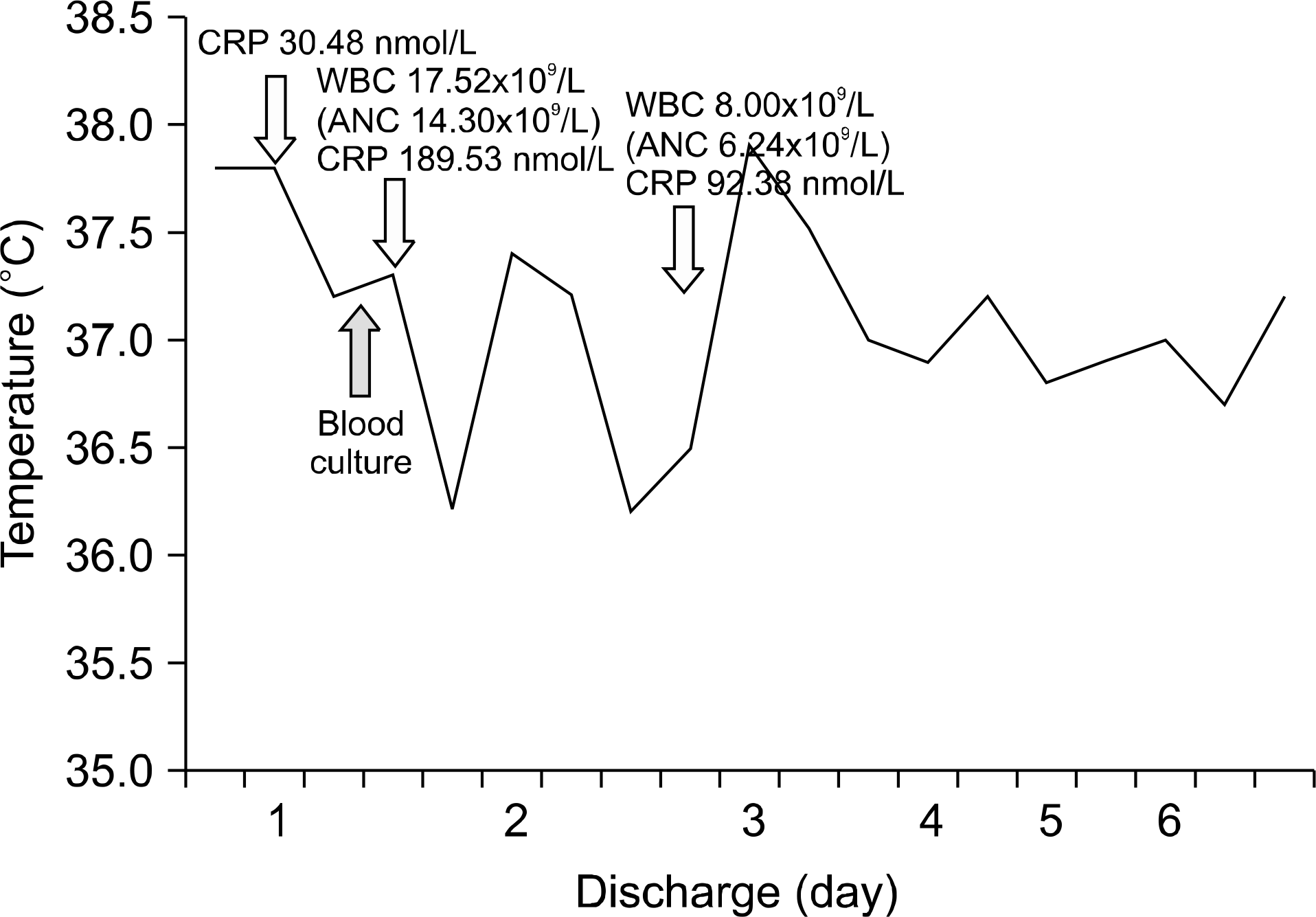
Fig. 2.
Colonial and microscopic morphology of Psychrobacter sanguinis. (A) Non-pigmented and non-hemolytic colonies with circular and smooth edges on a blood agar plate. (B) Gram-negative coccobacilli (Gram stain, ×1,000).
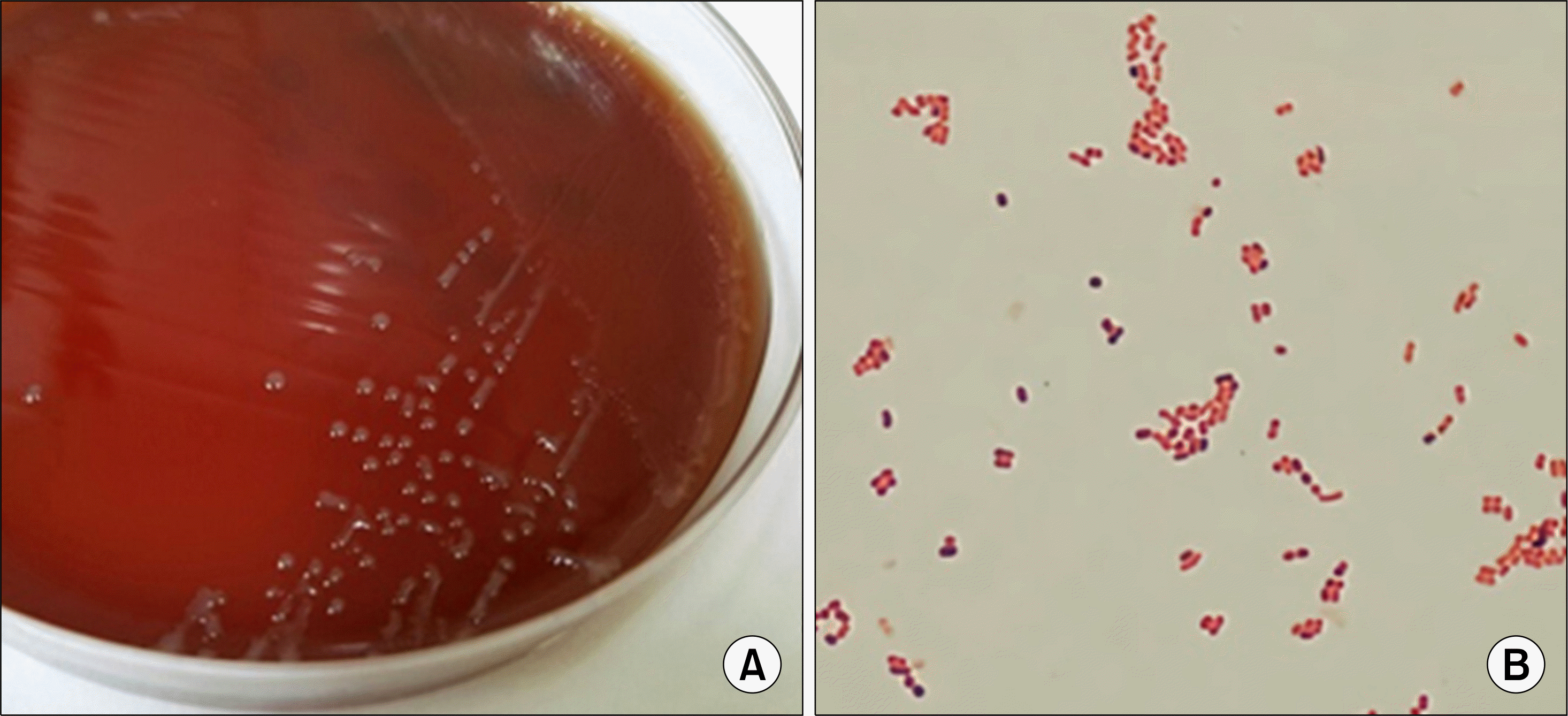
Fig. 3.
Unrooted neighbor-joining phylogenetic tree based on 16S rRNA sequences of Psychrobacter sanguinis and 20 similar organisms.
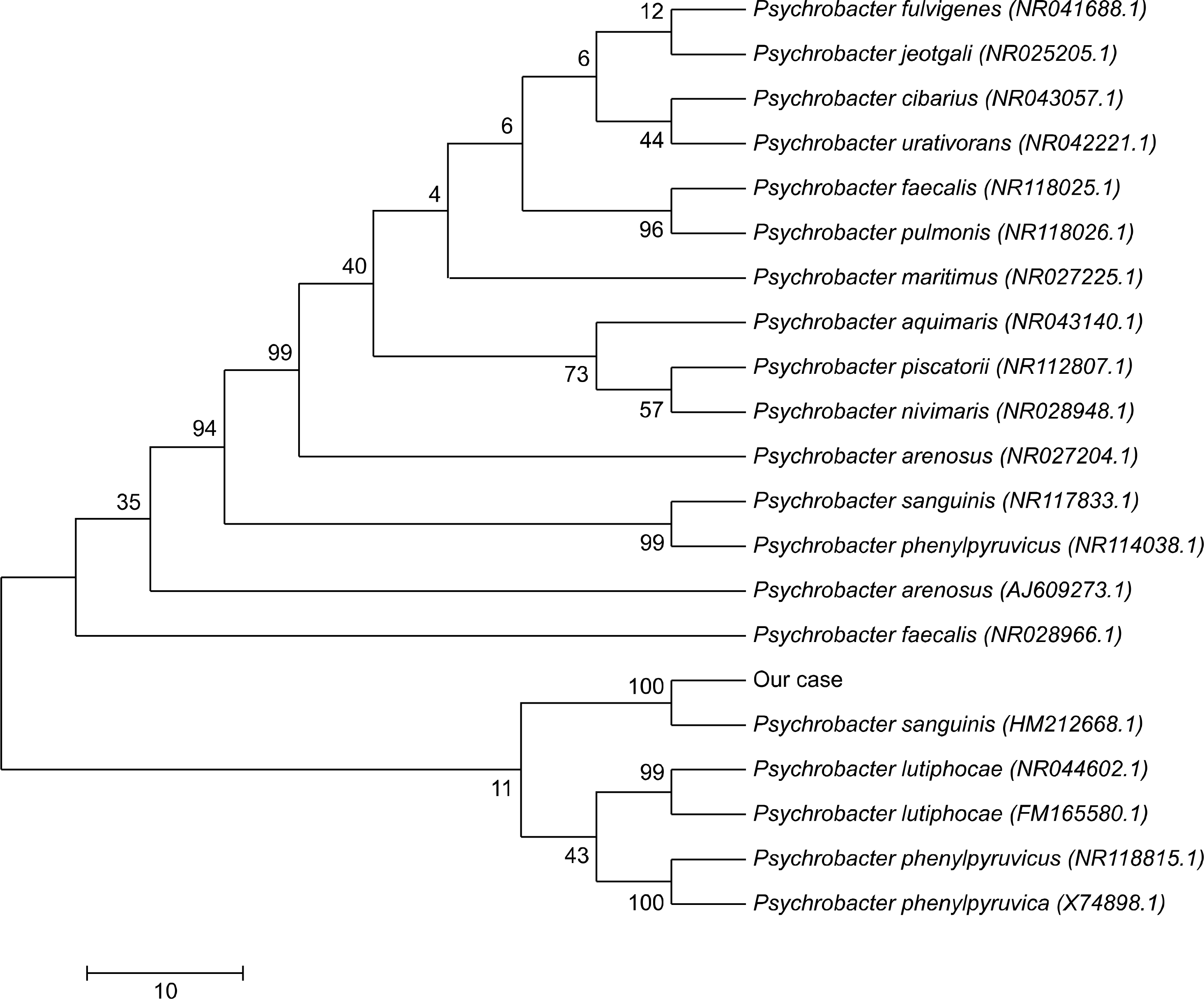
Table 1.
Summary of previously reported cases with Psychrobacter sanguinis from human specimens




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download