Abstract
Background
Candidemia has increased with an increasing number of people in the high risk group and so has become more important. This study was conducted to investigate the isolation rate of Candida species from candidemia patients and the change in rate of antifungal resistance.
Methods
At a single tertiary care hospital, 1,120 blood cultures positive for Candida species from 1997 to 2016 were investigated according to date of culture, gender, age, and hospital department.
Results
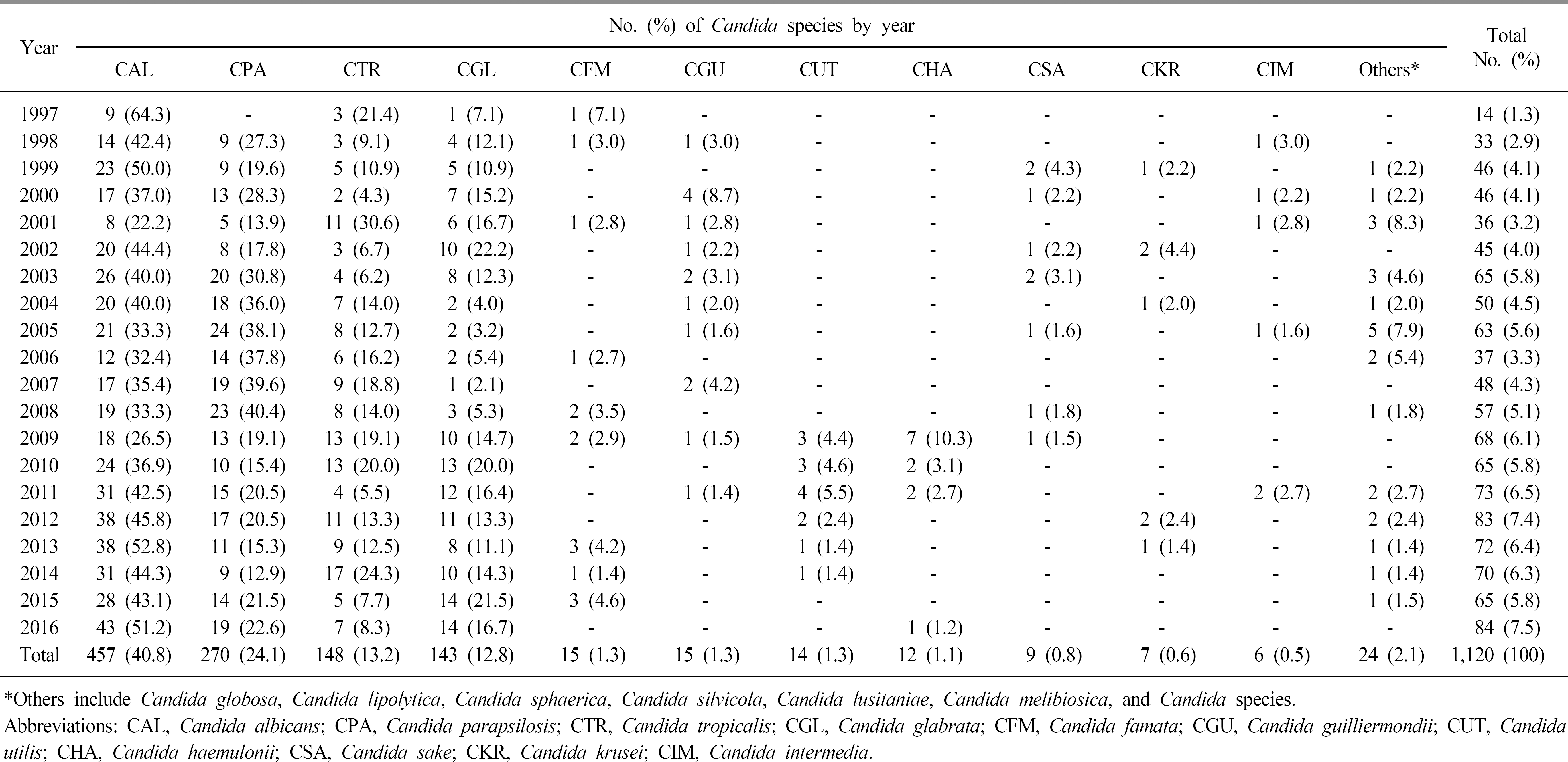
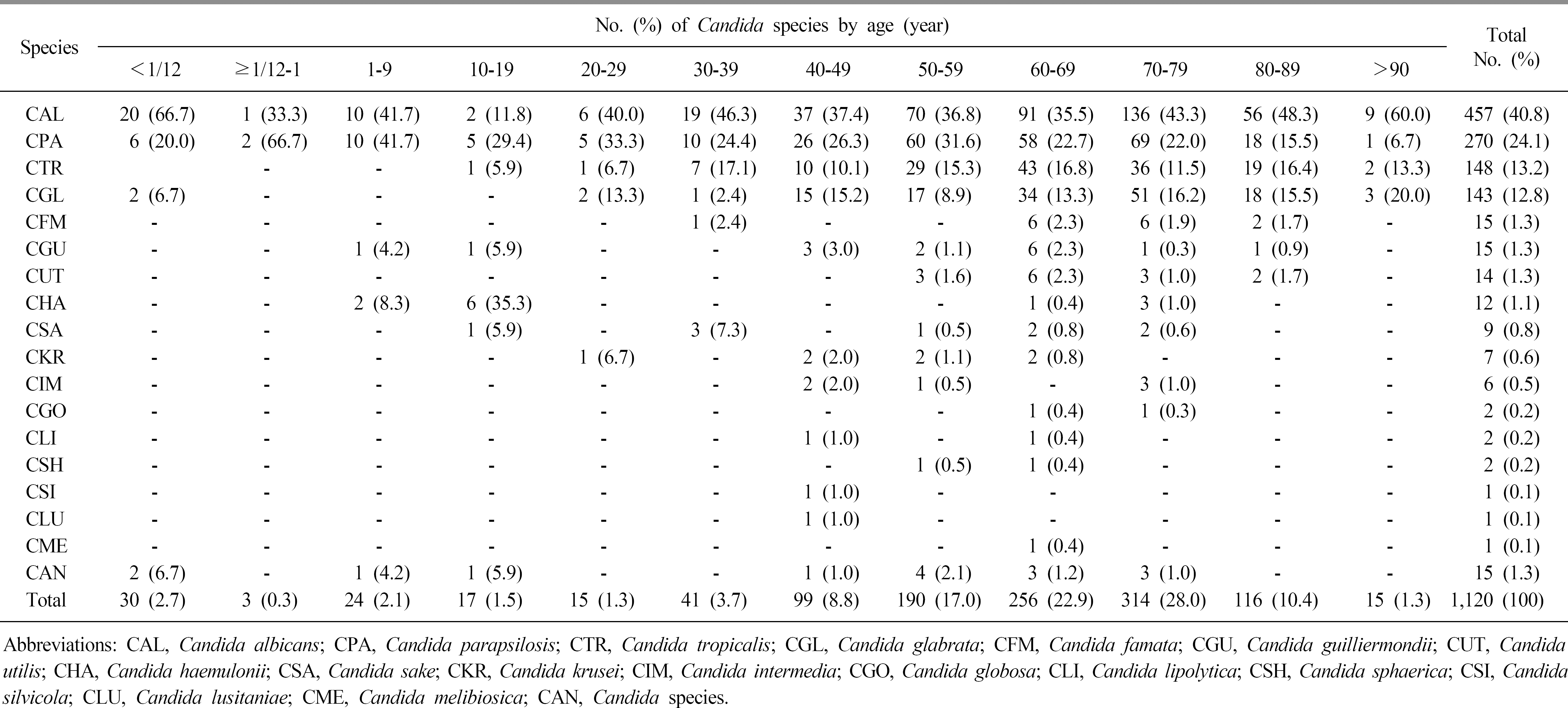
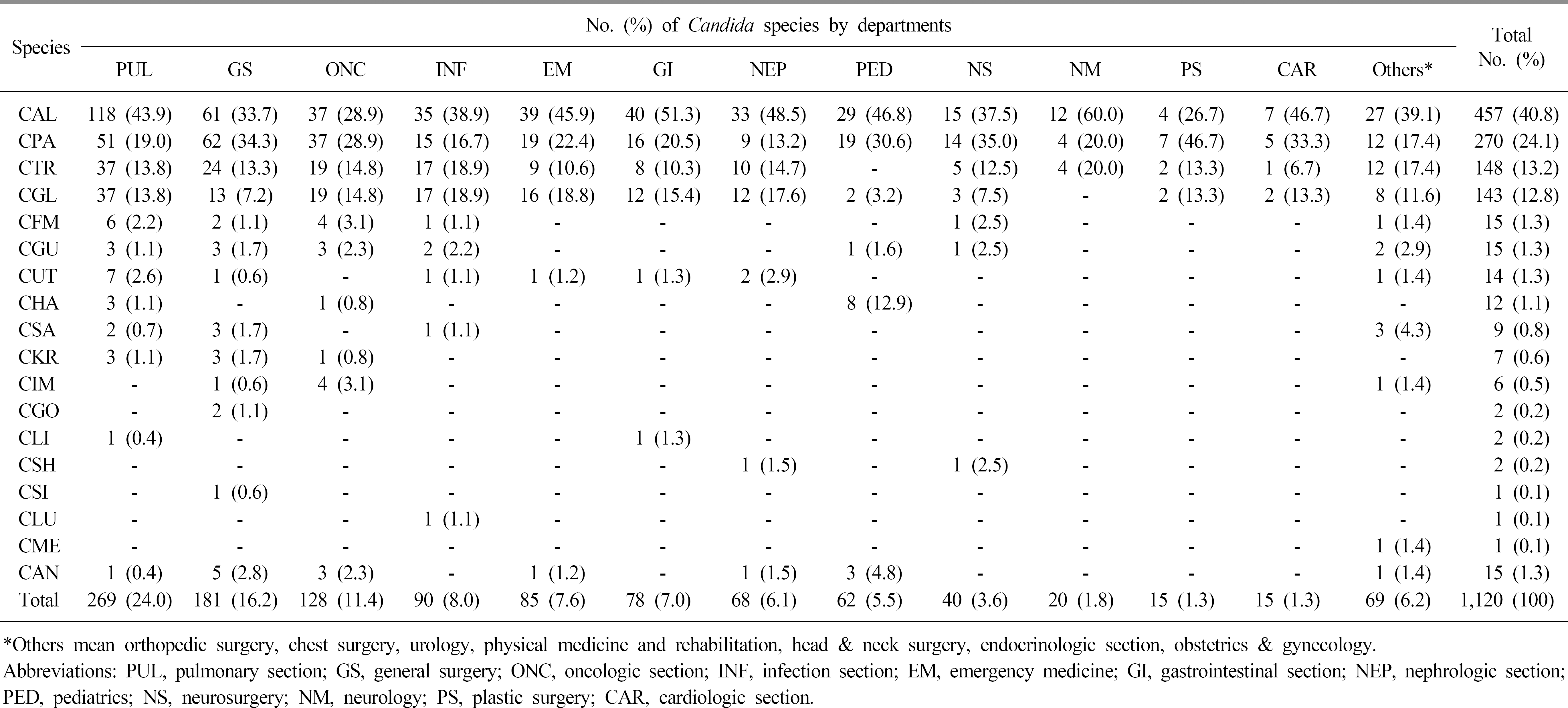
During the investigation period, the number of candidemia patients increased from 14 in 1997 to 84 in 2016. The most common organism identified during the two decades was Candida albicans (40.8%), followed by Candida parapsilosis (24.1%), Candida tropicalis (13.2%), and Candida glabrata (12.8%). C. glabrata was relatively common in females (45.5%) compared to males. The age group 40–89 years was more frequently infected than other age groups, and the most frequent isolates according to age group were C. albicans in neonate (66.7%), C. parapsilosis in 1–9-year-olds (41.7%), and C. glabrata in those aged ≥60 years (range; 13.3%-20.0%). According to the visited departments, C. albicans, C. glabrata, and Candida haemulonii were more common in medical departments, while C. parapsilosis was more common in surgical departments. In the antifungal susceptibility test, a rising trend of azole resistance among C. albicans and C. glabrata was observed in recent years.
Conclusion
In this study, it was confirmed that the isolation rate of Candida species in blood is different by age, gender, and hospital department, and the distribution of isolated Candida species changed over time. The resistance patterns of antifungal agents are also changing, and continuous monitoring and proper selection of antifungal agents are necessary.
Go to : 
References
1. Yang ZT, Wu L, Liu XY, Zhou M, Li J, Wu JY, et al. Epidemiology, species distribution and outcome of nosocomial Candida spp. bloodstream infection in Shanghai. BMC Infect Dis. 2014; 14:241.

2. Sanguinetti M, Posteraro B, Lass-Flörl C. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses. 2015; 58(Suppl 2):2–13.
3. Kao AS, Brandt ME, Pruitt WR, Conn LA, Perkins BA, Stephens DS, et al. The epidemiology of candidemia in two United States cities: results of a population-based active surveillance. Clin Infect Dis. 1999; 29:1164–70.

4. Nguyen MH, Peacock JE Jr, Tanner DC, Morris AJ, Nguyen ML, Snydman DR, et al. Therapeutic approaches in patients with candidemia. Evaluation in a multicenter, prospective, observational study. Arch Intern Med. 1995; 155:2429–35.

5. Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009; 48:1695–703.

6. Guery BP, Arendrup MC, Auzinger G, Azoulay E, Borges Sá M, Johnson EM, et al. Management of invasive candidiasis and candidemia in adult non-neutropenic intensive care unit patients: Part I. Epidemiology and diagnosis. Intensive Care Med. 2009; 35:55–62.

7. Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Ellis D, Tullio V, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida Species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol. 2010; 48:1366–77.
8. Gong X, Luan T, Wu X, Li G, Qiu H, Kang Y, et al. Invasive candidiasis in intensive care units in China: Risk factors and prognoses of Candida albicans and non-albicans Candida infections. Am J Infect Control. 2016; 44:e59–63.

9. Falagas ME, Roussos N, Vardakas KZ. Relative frequency of albicans and the various non-albicans Candida spp among candidemia isolates from inpatients in various parts of the world: a systematic review. Int J Infect Dis. 2010; 14:e954–66.

10. Park SD, Uh Y, Jang IH, Yoon KJ, Shin JH. Comparison of ATB FUNGUS 2 and VITEK-2 antifungal susceptibility (AST-YS01) tests for Candida species isolated from blood culture. Korean J Clin Microbiol. 2010; 13:114–20.

11. Won EJ, Shin JH, Lee WK, Koo SH, Kim SY, Park YJ, et al. Distribution of yeast and mold species isolated from clinical specimens at 12 hospitals in Korea during 2011. Ann Clin Microbiol. 2013; 16:92–100.

12. Chae MJ, Shin JH, Cho D, Kee SJ, Kim SH, Shin MG, et al. Antifungal susceptibilities and distribution of Candida species recovered from blood cultures over an 8-year period. Korean J Lab Med. 2003; 23:329–35.
13. Park JS, Uh Y, Jang IH, Yoon KJ. Analysis of blood culture results during 1986–1995. J Clin Pathol Qual Control. 1997; 19:367–75.
14. Diekema DJ, Messer SA, Brueggemann AB, Coffman SL, Doern GV, Herwaldt LA, et al. Epidemiology of candidemia: 3-year results from the emerging infections and the epidemiology of Iowa organisms study. J Clin Microbiol. 2002; 40:1298–302.

15. Tortorano AM, Peman J, Bernhardt H, Klingspor L, Kibbler CC, Faure O, et al. Epidemiology of candidaemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur J Clin Microbiol Infect Dis. 2004; 23:317–22.

16. Levin AS, Costa SF, Mussi NS, Basso M, Sinto SI, Machado C, et al. Candida parapsilosis fungemia associated with implantable and semi-implantable central venous catheters and the hands of healthcare workers. Diagn Microbiol Infect Dis. 1998; 30:243–9.

17. Pfaller M, Neofytos D, Diekema D, Azie N, Meier-Kriesche HU, Quan SP, et al. Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH AllianceⓇ) registry, 2004–2008. Diagn Microbiol Infect Dis. 2012; 74:323–31.

18. Shin JH, Lim WH, Shin DH, Suh SP, Ryang DW. Antifungal susceptibilities to fulconazole and itraconazole for Candida species recovered from blood cultures over a 5-year period. Korean J Infect Dis. 2000; 32:179–85.
19. Pfaller MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, et al. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J Clin Microbiol. 2008; 46:150–6.
Go to : 
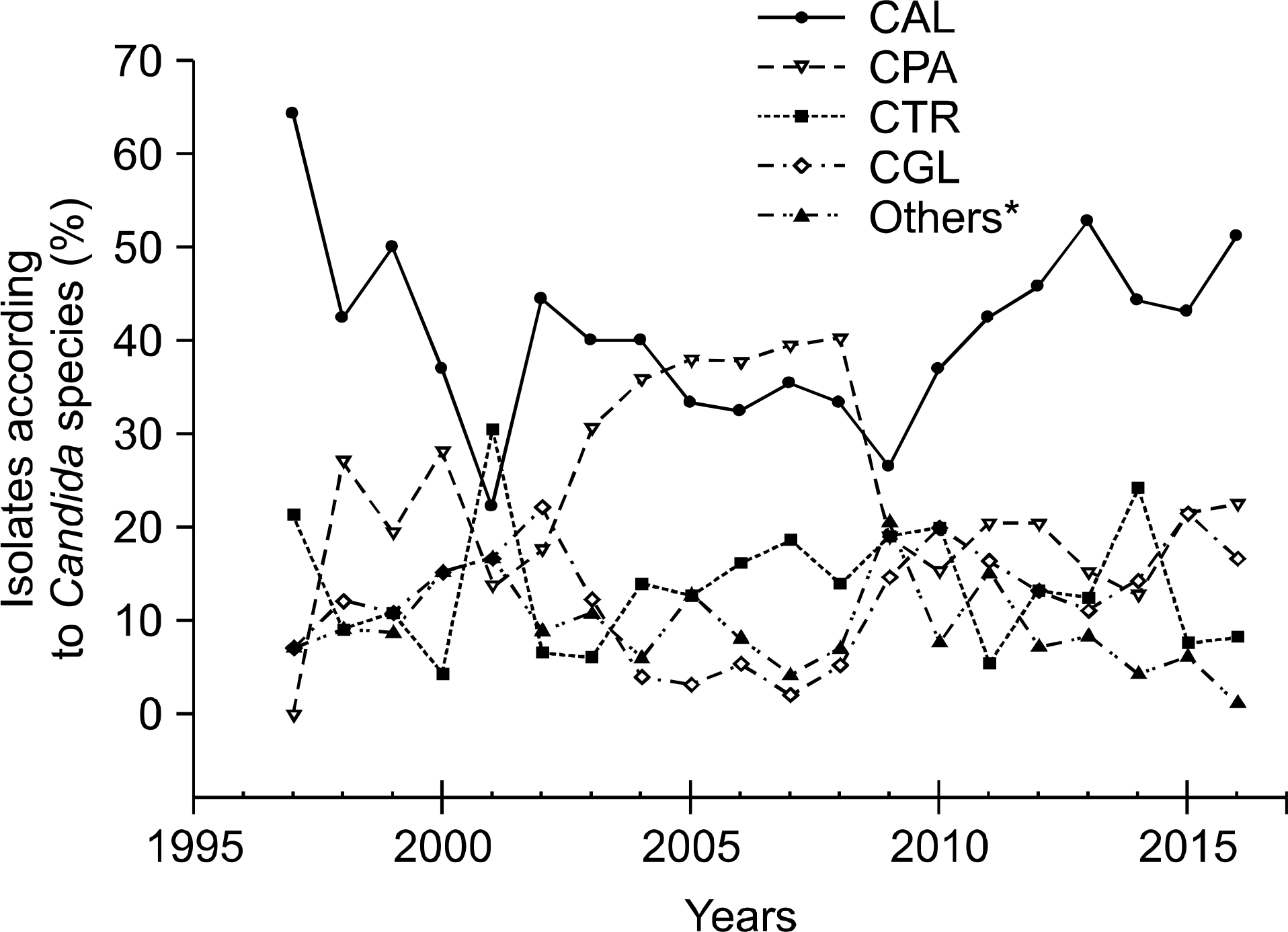 | Fig. 1.Isolation rate of Candida species in patients with candidemia during two decades (1997–2016). *Others includes Candida famata, Candida guilliermondii, Candida utilis, Candida haemulonii, Candida sake, Candida krusei, Candida intermedia, Candida globosa, Candida lipolytica, Candida sphaerica, Candida silvicola, Candida lusitaniae, Candida melibiosica, and Candida species. Abbreviations: CAL, Candida albicans; CPA, Candida parapsilosis; CTR, Candida tropicalis; CGL, Candida glabrata. |
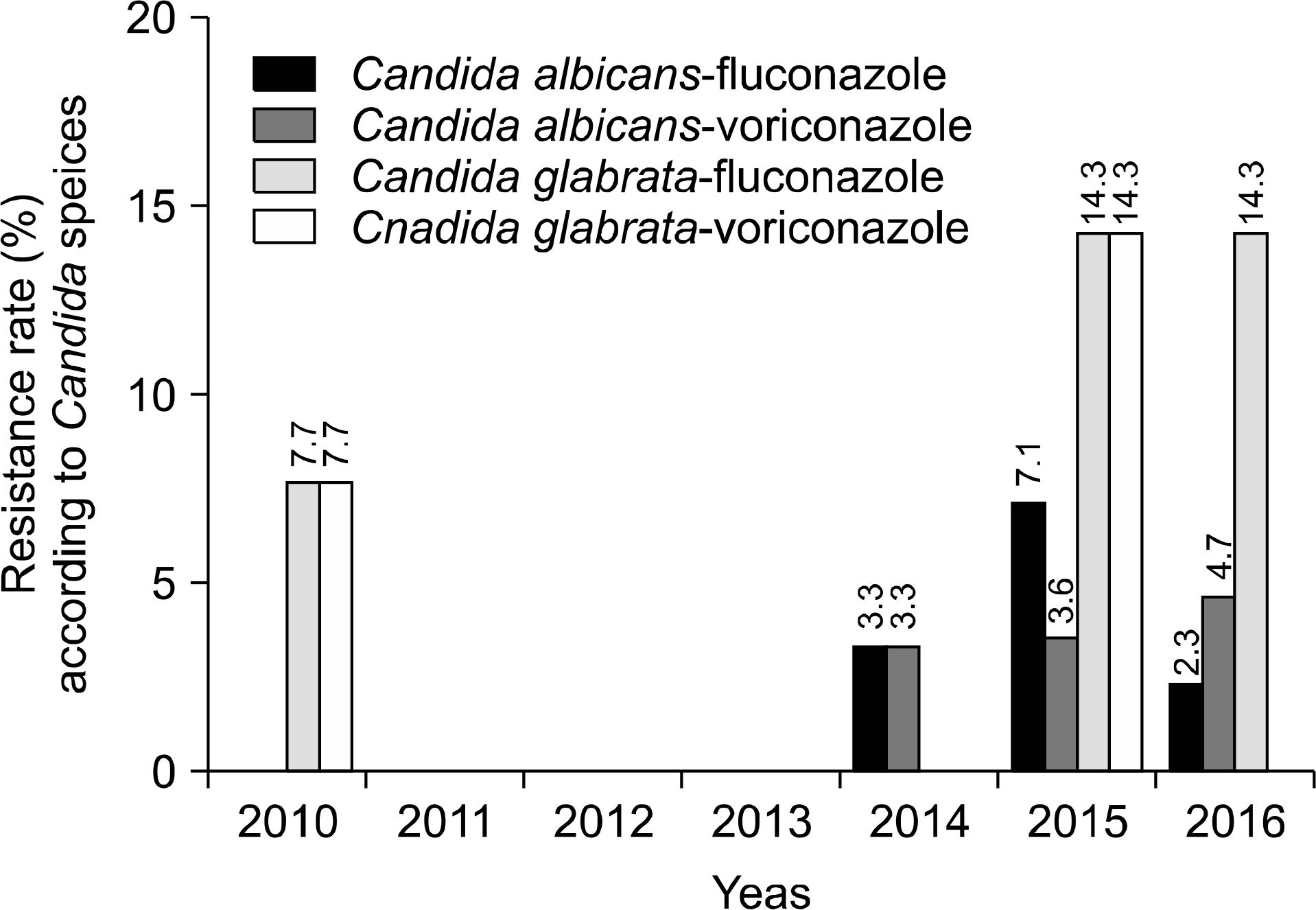 | Fig. 2.Resistance rate of C. albicans and C. glabrata for fluconazole and voriconazole during recent 7 years (2010–2016). |
Table 2.
Candida species according to gender
Table 5.
Candida species showing resistance to antifungal agents




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download