Abstract
Tuberculosis can occur in various organ systems and may present with diverse manifestations. We report an unusual case of mediastinal tuberculoma in a 3-month-old boy who presented to the hospital after experiencing fever, cough, and progressive pneumonia for two weeks. The chest computed tomography scan indicated a mediastinal mass suggesting lymphoma. However, histological analysis confirmed that the mass was caused by tuberculosis. The present report describes the delayed diagnosis of a disease due to an uncommon presentation. Misdiagnosing unusual cases of tuberculosis results in treatment delays and may lead to an increase in morbidity. Therefore, we suggest that tuberculosis should be included in the differential diagnosis for children presenting with a mediastinal mass, especially in areas with a high prevalence of tuberculosis.
Tuberculosis remains a major global health problem, responsible for ill health among millions of people each year. Tuberculosis ranks as the second leading cause of death from an infectious disease worldwide [1].
Tuberculosis can affect most organs of the human body and its clinical manifestations are diverse. It typically affects the lungs but can affect other sites as well. Sometimes tuberculosis occurs at unusual sites with unusual presentations that mimic other medical illnesses and cause diagnostic difficulties [2]. Misdiagnosis of unusual cases of tuberculosis results in treatment delays and may result in increased morbidity.
We report a case of unusual case of mediastinal tuberculoma in a 3-month-old boy whose diagnosis was delayed because of an uncommon presentation.
A 3-month-old boy presented to his local hospital with cough and fever. A chest radiograph (CXR) showed diffuse infiltration in both lungs. He was admitted and treated with multiple courses of intravenous antibiotics but the symptoms persisted and his condition deteriorated. On the 13th day of hospitalization, he developed respiratory distress. A CXR showed progression of infiltration in both lungs and consolidation affecting the right upper lobe. He was referred to a tertiary hospital for further evaluation. Upon examination he looked unwell and was afebrile. He was in respiratory distress (32 breaths/min). Hematological indices and inflammatory markers were normal. Cerebrospinal fluid (CSF) biochemistry and microbiology were unremarkable. There was no microbial growth from blood, CSF, or urine cultures. Immunizations were on the current schedule and his development was normal. Weight gain was in the 50th percentile for his age. There was no family history of note.
A CXR showed haziness in both lungs (Fig. 1A). A chest computed tomography (CT) scan showed a heterogeneous, mid-mediastinal mass suggesting lymphoma (Fig. 1B). There was a small amount of pleural fluid and no abnormalities in the lung parenchyma. Non-Hodgkin's lymphoma was the most likely cause. The differential diagnosis was a germ cell tumor and a neurogenic tumor.
On the 2nd day of hospitalization, he developed fever. After 4 days of intravenous antibiotics, he remained afebrile but continued to have mild tachypnea and a cough with persisting CXR changes. On the 4th day, he showed respiratory distress with subcostal retraction and required 0.5 L/min oxygen to maintain pulse oximetry saturations of 95%.
A chest CT scan on day 5 showed that the mid-mediastinal mass was larger with associated right upper lobe pneumonia. The right upper lobe bronchus was completely obstructed and the main bronchus was mildly compressed by the mass. Enlargement of the bilateral axillary lymph nodes was detected. There was no definitive evidence of pleural effusion.
On the 8th day, an elective incisional biopsy of the mass was performed. A pre-operative CXR showed subcutaneous emphysema. A right posterolateral thoracotomy found no adhesion in the pleural cavity and a complete lung fissure. A large mass was found filling the entire upper pleural cavity. A large central cyst containing necrotic caseous material was found in the mass and a large quantity of caseous purulent material was drained. Dissection was difficult as the trachea and superior vena cava were obliterated by inflammation of the mass.
The incisional specimen was sent for histopathological examination. The tissue specimen was a yellow/white color and 1.5 cc in volume. On frozen section analysis, multiple caseous nodules were found. The permanent section showed chronic granulomatous inflammation and necrosis compatible with tuberculosis (Fig. 2A). Ziehl-Neelsen stains were positive for acid-fast bacilli (AFB) (Fig. 2B). The purulent material from the mass also showed AFB on Ziehl-Neelsen staining (Fig. 2C). Although some reactive lymphocyte proliferation was seen in the areas of inflammation, no evidence of lymphoma was found.
On the 11th day, sputum smears were positive for AFB on Ziehl-Neelsen staining. The interferon-gamma release assays (Oxford Immunotec, Abingdon, UK) were positive. A polymerase chain reaction assay (LG Life Science, Daejon, Korea) on gastric juice was positive for Mycobacterium tuberculosis. Mycobacterial culture using 3% Ogawa media (Shinyang Chemical Co., Ltd, Seoul, Korea) on pleural fluid were positive after 4 weeks of inoculation. The culture isolate were confirmed using MPT 64 test (Standard Diagnostics, Seoul, Korea). For drug susceptibility testing, the culture isolate was referred to the Korean Institute of Tuberculosis. The strain was susceptible to all first line anti-tuberculosis drugs.
Anti-tuberculosis medications were administrated on the 11th day. Because of diagnostic delays, 3 weeks elapsed between the initial presentation at the local hospital and the administration of anti-tuberculosis medications. To identify the possible transmission routes, further investigations were conducted on family members and close contacts. However, no evidence of infection was found.
At the 1-year follow-up, fever and cough had subsided, weight was above the 50th percentile, and complete radiological resolution was seen on the CXR.
In this case, tuberculosis is present as pneumonia with pleural effusion in the pulmonary region, and mass in the extrapulmonary region. The most common type of tuberculosis in children is pulmonary tuberculosis but extrapulmonary tuberculosis occurs in approximately 20–30% of all cases. The common extrapulmonary involvement of tuberculosis in children is lymphatic disease and meningeal disease following primary tuberculosis [3]. Tuberculosis presenting as a mediastinal mass is rare and manifests with different presentations. Current knowledge about these conditions is mainly based on case reports. For example, one case report [4] described a 7-month-old infant who presented with breathlessness. The CT scan showed an irregularly calcified superior mediastinal mass that was initially mistaken as a neuroblastoma. However, the histological analysis showed that the mass was caused by tuberculosis. Another report described a 3-month-old boy with a tuberculous mediastinal mass causing stridor and obstructive emphysema [5]. A CT scan showed a mass compressing the carina and left bronchus. Another case [6] described a 10-month-old girl who presented with a possible inhaled foreign body. She underwent a thoracotomy and excision of the mass but the histological analysis showed tuberculosis. In our case, non-Hodgkin's lymphoma was the most likely diagnosis before histological confirmation. The differential diagnosis included a germ cell tumor and a neurogenic tumor. Prior to excision and histological confirmation of the mass, radiological testing mistakenly indicated a possible malignancy or inhaled foreign body in all of these cases.
In children, non-Hodgkin lymphoma, Hodgkin's disease, and neuroblastoma are common causes of mediastinal masses and potential differential diagnoses include thymic or thyroid masses [789]. In neonates and infants, cystic malformations on the mediastinal structures, such as cystic hygromas, can also be included [10].
Since the patient is an infant, disseminated bacille Calmette-Guérin (BCG) disease after vaccination also should be considered. Disseminated BCG disease is uncommon but one of the serious complication of vaccination especially in the high risk group such as children younger than 2 years old or immunocompromised infants [11]. In this case, because the patient was susceptible to pyrazinamide, M. bovis which is uniformly resistant to pyrizinamide is unlikely to be the cause of infection.
Any patient with pneumonia, pleural effusion, or a cavitary or mass lesion in the lung that does not improve with standard antibacterial therapy should be evaluated for tuberculosis. Misdiagnosis of tuberculosis results in treatment delays and may result in increased morbidity [12]. In addition, tuberculosis infected children under 5 years old are at high risk of developing more severe tuberculous [13]. However, a large number of tuberculosis related deaths and morbidity can be prevented with early diagnosis and timely intervention with anti-tuberculosis therapy.
As this case demonstrates, tuberculosis can present in unusual ways that may cause a delayed diagnosis. To prevent this delay, we suggest that any patient with pneumonia or pleural effusion that does not improve with standard antibacterial therapy should be evaluated for tuberculosis. Also tuberculosis should be included in the differential diagnosis for children presenting with mediastinal masses, especially in areas with an intermediate-to-high prevalence of tuberculosis. Early detection of these uncommon types of tuberculosis may lead to better outcomes.
Figures and Tables
Fig. 1
Radiological findings of the patient. (A) The plain chest radiograph taken upon admission showed haziness in both lungs. (B) The initial chest CT scan showed a large mediastinal mass compressing the main bronchus.
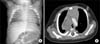
Fig. 2
Histopathological findings of the surgical specimens. (A) The permanent section showed chronic granulomatous inflammation and necrosis compatible with tuberculosis infection (hematoxylin and eosin stain, ×40). (B) The surgical specimens showed acid-fast bacilli (Ziehl-Neelsen staining, ×1,000). (C) The purulent material from the inside of mass showed acid-fast bacilli (Ziehl-Neelsen staining, ×1,000).

ACKNOWLEDGMENTS
This work was supported by the annual clinical research grant from Pusan National University Yangsan Hospital.
References
1. WHO. Global Tuberculosis Report 2014. Geneva: World Health Organization;2014.
2. Al-Tawfiq JA. Multifocal systemic tuberculosis: the many faces of an old nemesis. Med Sci Monit. 2007; 13:CS56–CS60.
3. Ussery XT, Valway SE, McKenna M, Cauthen GM, McCray E, Onorato IM. Epidemiology of tuberculosis among children in the United States: 1985 to 1994. Pediatr Infect Dis J. 1996; 15:697–704.
4. Gillies MJ, Farrugia MK, Lakhoo K. An unusual cause of a superior mediastinal mass in an infant. Pediatr Surg Int. 2008; 24:485–486.
5. De Ugarte DA, Shapiro NL, Williams HL. Tuberculous mediastinal mass presenting with stridor in a 3-month-old child. J Pediatr Surg. 2003; 38:624–625.
6. Ahmed A, Mirza S, Rothera MP. Mediastinal tuberculosis in a 10-month-old child. J Laryngol Otol. 2001; 115:161–163.
7. Simpson I, Campbell PE. Mediastinal masses in childhood: a review from a paediatric pathologist's point of view. Prog Pediatr Surg. 1991; 27:92–126.
8. Jaggers J, Balsara K. Mediastinal masses in children. Semin Thorac Cardiovasc Surg. 2004; 16:201–208.
9. Ranganath SH, Lee EY, Restrepo R, Eisenberg RL. Mediastinal masses in children. AJR Am J Roentgenol. 2012; 198:W197–W216.
10. Grosfeld JL, Skinner MA, Rescorla FJ, West KW, Scherer LR 3rd. Mediastinal tumors in children: experience with 196 cases. Ann Surg Oncol. 1994; 1:121–127.
11. Talbot EA, Perkins MD, Silva SF, Frothingham R. Disseminated bacille Calmette-Guérin disease after vaccination: case report and review. Clin Infect Dis. 1997; 24:1139–1146.
12. Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008; 8:15.
13. Palomino JC, Leão SC, Ritacco V. Tuberculosis 2007; from basic science to patient care. São Paulo: Amedeo Challenge;2007.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download