Abstract
Background
The recovery of bacteria from blood can be affected by many factors. Standardization of blood culture methods is important for reliability. Herein, we aimed to investigate blood culture protocols in Korea.
Methods
We performed a multicenter survey with a questionnaire about blood culture practices, which was sent by email to directors and clinical physicians in charge of clinical microbiology laboratories in May 2014. Total data from 18 participating hospitals were analyzed to be used as current baseline data, which is necessary to optimize blood culture protocols.
Results
Many laboratories included recommended blood volume, which is a major factor for bacteria recovery rate. This varied across participating laboratories. For adults, blood sampling of 10 mL was recommended by 10 laboratories and 20 mL was recommended by 5 laboratories. For children who weighed 14–36 kg and less than 14 kg, blood sampling of 10 mL (n=8) and 5 mL (n=7) was recommended, respectively. For neonates, less than 1 mL was recommended by 12 laboratories.
Go to : 
References
1. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013; 39:165–228.

2. Kim S. Development of blood culture and quality improvement. Ann Clin Microbiol. 2013; 16:153–61.

3. Versalovic J, Carroll KC, et al. eds. Manual of Clinical Microbiology. 8th ed.American Society for Microbiology;2010. p. 15–26.
4. CLSI. Principles and procedures for blood cultures: approved guideline. CLSI document M47-A. Wayne, PA: Clinical Laboratory Standard Institute;2007.
5. Public Health England. Standards for microbiology investigations (SMI). https://www.gov.uk/government/collections/standards-for-microbiology-investigations-smi. [Online] (last visited on 23 August 2016).
6. Shin JH, Song SA, Kim MN, Lee NY, Kim EC, Kim S, et al. Comprehensive analysis of blood culture performed at nine university hospitals in Korea. Korean J Lab Med. 2011; 31:101–6.

7. Kang H, Kim SC, Kim S. Comparison of chlorhexidine-alcohol and povidone-iodine for skin antisepsis and the effect of increased blood volume in blood culture. Korean J Clin Microbiol. 2012; 15:37–42.

8. Koh EH, Lee DH, Kim S. Effects of preincubating blood culture bottles at 37 o C during the night shift and of collected blood volume on time to detection and days to final report. Ann Clin Microbiol. 2014; 17:14–9.
9. Shin JH, Song SA, Kim MN, Kim S. Nationwide survey of blood culture performance regarding Skin disinfection, blood collection and laboratory procedures. Korean J Clin Microbiol. 2011; 14:91–6.

10. Howie SR. Blood sample volumes in child health research: review of safe limits. Bull World Health Organ. 2011; 89:46–53.

11. Song SA, Kim JH, Shin JH, Kim SH, Lee NY, Kim MN, et al. Clinical usefulness of routine use of anaerobic blood culture bottle. Ann Clin Microbiol. 2014; 17:35–41.

12. Roe DE, Finegold SM, Citron DM, Goldstein EJ, Wexler HM, Rosenblatt JE, et al. Multilaboratory comparison of growth characteristics for anaerobes, using 5 different agar media. Clin Infect Dis. 2002; 35(Suppl 1):S36–9.

13. Gunn BA. Chocolate agar, a differential medium for gram-positive cocci. J Clin Microbiol. 1984; 20:822–3.

Go to : 
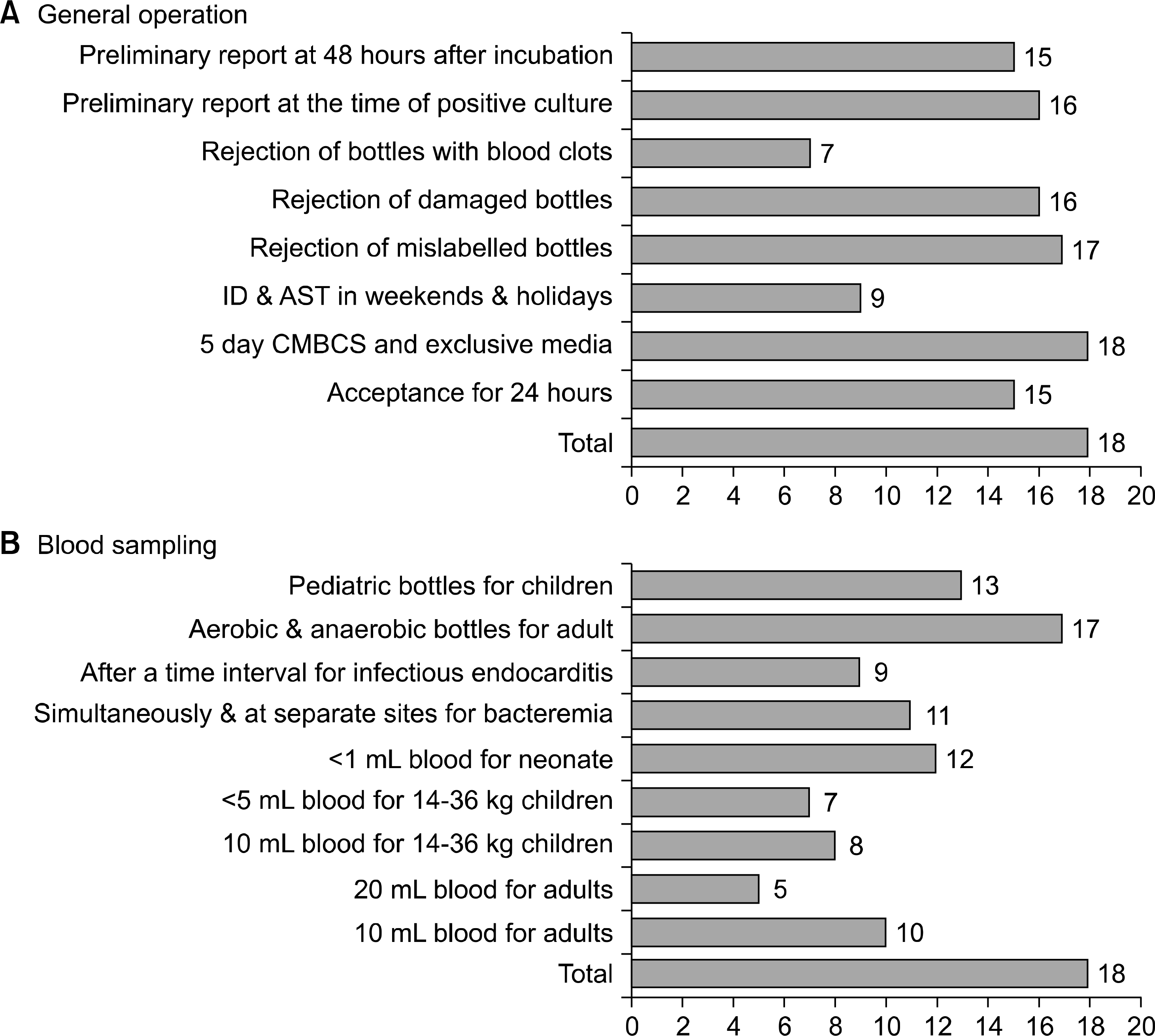 | Fig. 1.The blood culture protocol and procedures in 18 microbiology laboratories in Korea. Abbreviations: QC, quality control; CNS, coagulase-ne-gative staphylococci; PEA, phenylethyl alcohol blood agar; BA, blood agar; AST, antimicrobial susceptibility test; ID, identification; CMBCS, continuous monitoring blood culture system. |
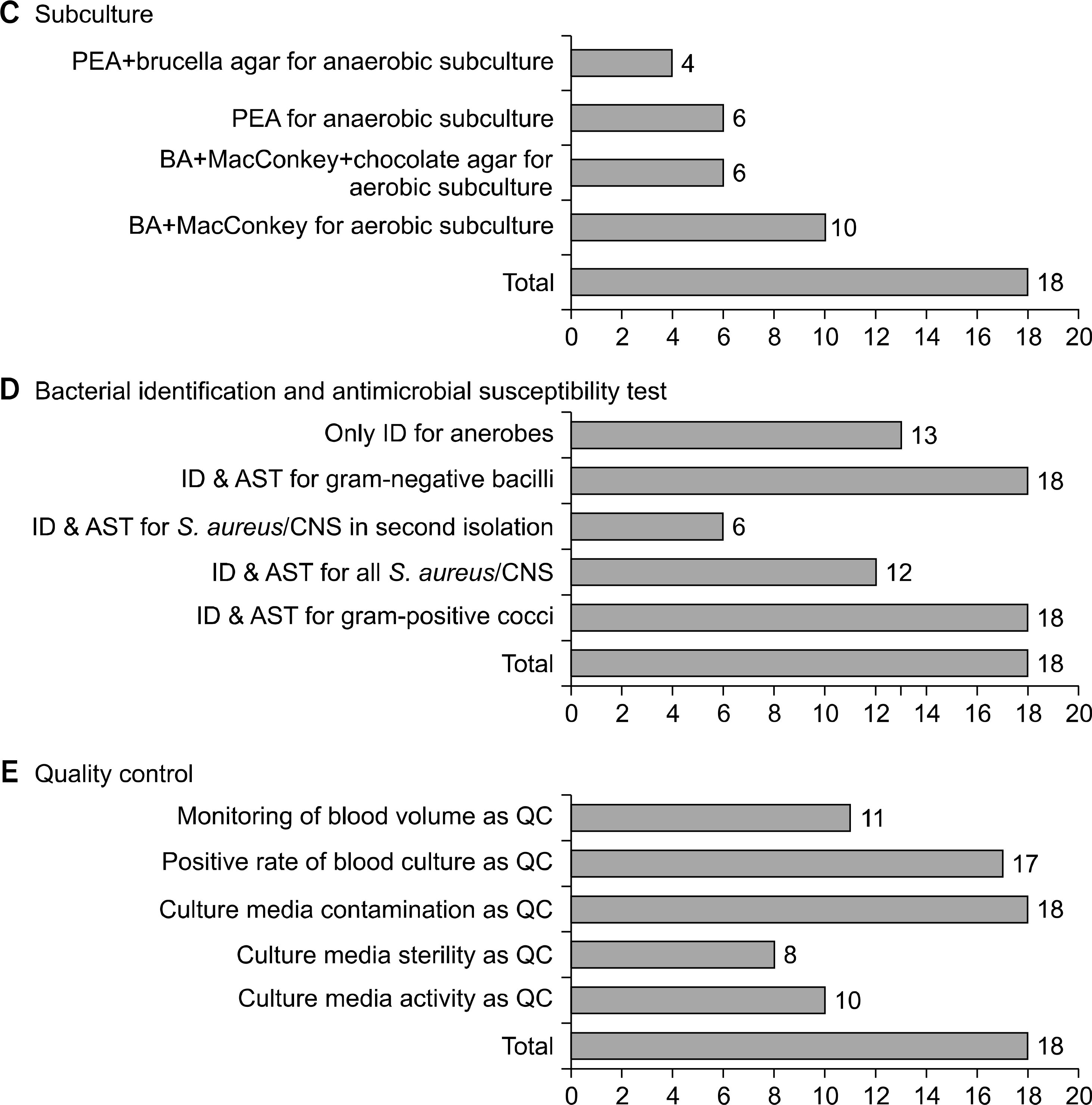 | Fig. 1.The blood culture protocol and procedures in 18 microbiology laboratories in Korea. Abbreviations: QC, quality control; CNS, coagulase-ne-gative staphylococci; PEA, phenylethyl alcohol blood agar; BA, blood agar; AST, antimicrobial susceptibility test; ID, identification; CMBCS, continuous monitoring blood culture system. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download