Abstract
Background
We evaluated the carbapenem inactivation method (CIM) compared with the modified Hodge test (MHT) for the detection of carbapene-mase-producing Gram-negative bacilli.
Methods
A total of 61 isolates of carbapenemase-producing Enterobacteriaceae (CPE: 14 KPC, 7 GES-5, 8 NDM-1, 9 VIM-2, 9 IMP-1, and 14 OXA-48-like), 34 isolates of metallo-β-lactamase (MBL)-producing Pseudomonas spp. (14 VIM-2 and 20 IMP-6), and 70 carbapenem-nonsusceptible carbapenemase-negative isolates were included. The CIM and MHT were performed for all of the isolates. To perform the CIM, a meropenem disk was incubated with a suspension of the isolate to be tested and then on Mueller-Hinton agar with the Escherichia coli ATCC 29522 strains. The absence of an inhibition zone indicates presence of a carbapenemase. The presence of a clearing zone indicates lack of a carbapenemase.
Results
The total sensitivity and specificity of CIM (96% sensitivity and 100% specificity) in carbapenem-nonsusceptible Enterobacteriaceae and Pseudomonas spp. were better than those of the MHT (77% sensitivity and 94% specificity). The interpretation of CIM results was easy, with no or <20 mm inhibition zones indicating positivity and >20 mm inhibition zones indicating negative carbapenemase activity.
Go to : 
References
1. Patel JB, Rasheed JK, Kitchel B. Carbapenemases in Enterobacteriaceae: activity, epidemiology, and laboratory detection. Clin Microbiol Newsletter. 2009; 31:55–62.

2. Girlich D, Poirel L, Nordmann P. Value of the modified Hodge test for detection of emerging carbapenemases in Enterobacteriaceae. J Clin Microbiol. 2012; 50:477–9.

3. Jeong SH, Song W, Bae IK, Kim HS, Kim JS, Park MJ, et al. Broth microdilution methods using B-lactamase inhibitors for the identification of Klebsiella pneumoniae carbapenemases and metallo-β-lactamases in Gram-negative bacilli. Ann Clin Lab Sci. 2014; 44:49–55.
4. Papagiannitsis CC, Študentová V, Izdebski R, Oikonomou O, Pfeifer Y, Petinaki E, et al. Matrix-assisted laser desorption ionization-time of flight mass spectrometry meropenem hydrolysis assay with NH4 HCO3, a reliable tool for direct detection of carbapenemase activity. J Clin Microbiol. 2015; 53:1731–5.
5. CLSI. Performance standards for antimicrobial susceptibility testing. CLSI document M100-S23. Wayne, PA: Clinical and Laboratory Standards Institute;2013.
6. Anderson KF, Lonsway DR, Rasheed JK, Biddle J, Jensen B, McDougal LK, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol. 2007; 45:2723–5.
7. Pasteran F, Mendez T, Guerriero L, Rapoport M, Corso A. Sensitive screening tests for suspected class A carbapenemase production in species of Enterobacteriaceae. J Clin Microbiol. 2009; 47:1631–9.
8. Pasteran F, Mendez T, Rapoport M, Guerriero L, Corso A. Controlling false-positive results obtained with the Hodge and Masuda assays for detection of class a carbapenemase in species of Enterobacteriaceae by incorporating boronic acid. J Clin Microbiol. 2010; 48:1323–32.
9. Carvalhaes CG, Picão RC, Nicoletti AG, Xavier DE, Gales AC. Cloverleaf test (modified Hodge test) for detecting carbapenemase production in Klebsiella pneumoniae: be aware of false positive results. J Antimicrob Chemother. 2010; 65:249–51.

10. Doumith M, Ellington MJ, Livermore DM, Woodford N. Molecular mechanisms disrupting porin expression in ertapenem-resistant Klebsiella and Enterobacter spp. clinical isolates from the UK. J Antimicrob Chemother. 2009; 63:659–67.

11. Hirsch EB and Tam VH. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J Antimicrob Chemother. 2010; 65:1119–25.
12. van der Zwaluw K, de Haan A, Pluister GN, Bootsma HJ, de Neeling AJ, Schouls LM. The carbapenem inactivation method (CIM), a simple and low-cost alternative for the Carba NP test to assess phenotypic carbapenemase activity in gram-negative rods. PLoS One. 2015; 10:e0123690.

13. Jeong S, Kim JO, Jeong SH, Bae IK, Song W. Evaluation of peptide nucleic acid-mediated multiplex realtime PCR kits for rapid detection of carbapenemase genes in gram-negative clinical isolates. J Microbiol Methods. 2015; 113:4–9.

14. Song W, Hong SG, Yong D, Jeong SH, Kim HS, Kim HS, et al. Combined use of the modified Hodge test and carbapenemase inhibition test for detection of carbapenemase-producing Enterobacteriaceae and metallo-β-lactamase-producing Pseudomonas spp. Ann Lab Med. 2015; 35:212–9.

15. Tijet N, Patel SN, Melano RG. Detection of carbapenemase activity in Enterobacteriaceae: comparison of the carbapenem inactivation method versus the Carba NP test. J Antimicrob Chemother. 2016; 71:274–6.
16. Yamada K, Kashiwa M, Arai K, Nagano N, Saito R. Comparison of the modified-hodge test, Carba NP test, and carbapenem inactivation method as screening methods for carbapenemase-producing Enterobacteriaceae. J Microbiol Methods. 2016; 128:48–51.
17. Nordmann P, Poirel L, Dortet L. Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2012; 18:1503–7.
Go to : 
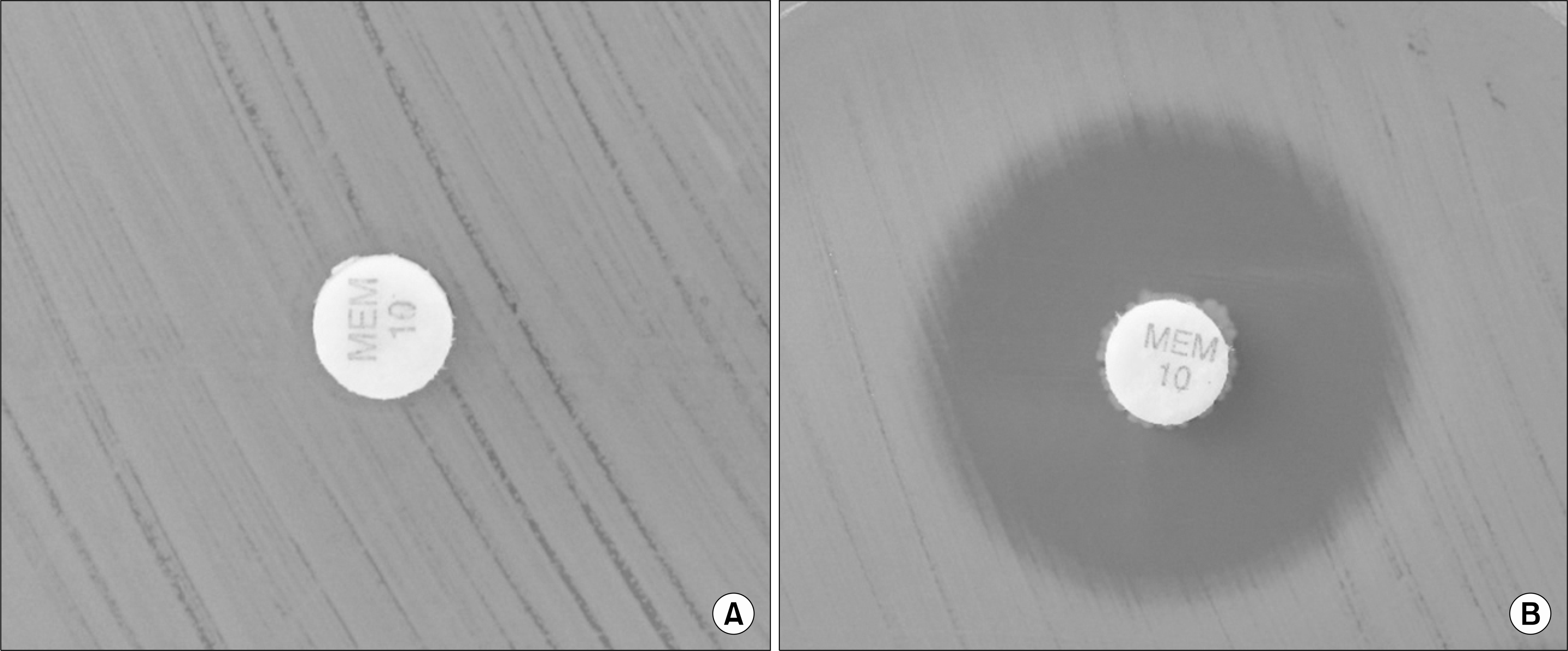 | Fig. 1.Interpretation of carbapenem inactivation method (CIM). The positive results showed the absence of an inhibition zone (A) and the negative results appeared >20 mm of inhibition zone diameter (B). |
Table 1.
Results of the CIM and MHT in carbapenem-nonsusceptible Enterobacteriaceae isolates
Table 2.
Characteristics of the CIM and MHT in carbapenem-nonsusceptible Pseudomonas spp. isolates
Table 3.
Sensitivity and specificity of the CIM and MHT in carbapenem-nonsusceptible Gram-negative bacilli




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download