Abstract
Mycobacterium abscessus was isolated from cultures of seven blood samples from a 64-year-old diabetic female who was admitted due to steroid-unrespon-sive adrenal insufficiency. The isolates were difficult to identify using the conventional commercial systems, VITEK 2 (bioMérieux, France) or MicroScan (Siemens Healthcare Diagnostics, USA), but were rapidly identified as M. abscessus by a matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS)-based Bruker Biotyper system (Bruker Daltonics, USA). Identification of M. abscessus was confirmed by a reverse hybridization-based assay (Genotype Mycobacterium CM/AS 12, Hain Lifescience) and direct sequencing of a heat-shock protein gene. After removal of her central venous catheter, the patient was successfully treated with a combination therapy comprising clarithromycin, amikacin, cefoxitin, and imipenem. Our findings demonstrate that MALDI-TOF MS can facilitate rapid and accurate identification of M. abscessus from blood cultures, which enables prompt administration of appropriate therapy following catheter removal.
References
1. Wagner D and Young LS. Nontuberculous mycobacterial infections: a clinical review. Infection. 2004; 32:257–70.

2. Brown-Elliott BA and Wallace RJ Jr. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev. 2002; 15:716–46.

3. Lee MR, Sheng WH, Hung CC, Yu CJ, Lee LN, Hsueh PR. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis. 2015; 21:1638–46.
4. Lee MR, Ko JC, Liang SK, Lee SW, Yen DH, Hsueh PR. Bacteraemia caused by Mycobacterium abscessus subsp. abscessus and M. abscessus subsp. bolletii: clinical features and susceptibilities of the isolates. Int J Antimicrob Agents. 2014; 43:438–41.

5. El Helou G, Viola GM, Hachem R, Han XY, Raad II. Rapidly growing mycobacterial bloodstream infections. Lancet Infect Dis. 2013; 13:166–74.

6. Kim HY, Kook Y, Yun YJ, Park CG, Lee NY, Shim TS, et al. Proportions of Mycobacterium massiliense and Mycobacterium bolletii strains among Korean Mycobacterium chelonae-Mycobac-terium abscessus group isolates. J Clin Microbiol. 2008; 46:3384–90.
7. Mediavilla-Gradolph MC, De Toro-Peinado I, Bermúdez-Ruiz MP, García-Martínez Mde L, Ortega-Torres M, Montiel Quezel-Guerraz N, et al. Use of MALDI-TOF MS for identification of nontuberculous Mycobacterium species isolated from clinical specimens. Biomed Res Int. 2015; 2015:854078.
8. Mareković I, Bošnjak Z, Jakopović M, Boras Z, Janković M, Popović-Grle S. Evaluation of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry in identification of nontuberculous mycobacteria. Chemotherapy. 2016; 61:167–70.

9. Telenti A, Marchesi F, Balz M, Bally F, Böttger EC, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993; 31:175–8.

10. CLSI. Susceptibility testing of mycobacteria, nocardiae and other aerobic actinomycetes: approved standard-second edition. CLSI document M24-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2011.
11. Teng SH, Chen CM, Lee MR, Lee TF, Chien KY, Teng LJ, et al. Matrix-assisted laser desorption ionization-time of flight mass spectrometry can accurately differentiate between Mycobacterium masilliense (M. abscessus subspecies bolletti) and M. abscessus (sensu stricto). J Clin Microbiol. 2013; 51:3113–6.
12. Panagea T, Pincus DH, Grogono D, Jones M, Bryant J, Parkhill J, et al. Mycobacterium abscessus complex identification with matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2015; 53:2355–8.

13. De Groote MA. Disease resulting from contaminated equipment and invasive procedures. Pedley S, Bartram J, Rees G, Dufour A, Cotruvo J, editors. Pathogenic Mycobacteria in Water: A Guide to Public Health Consequences, Monitoring and Management. Padstow, Cornwall: TJ International;2004. p. 131–42.
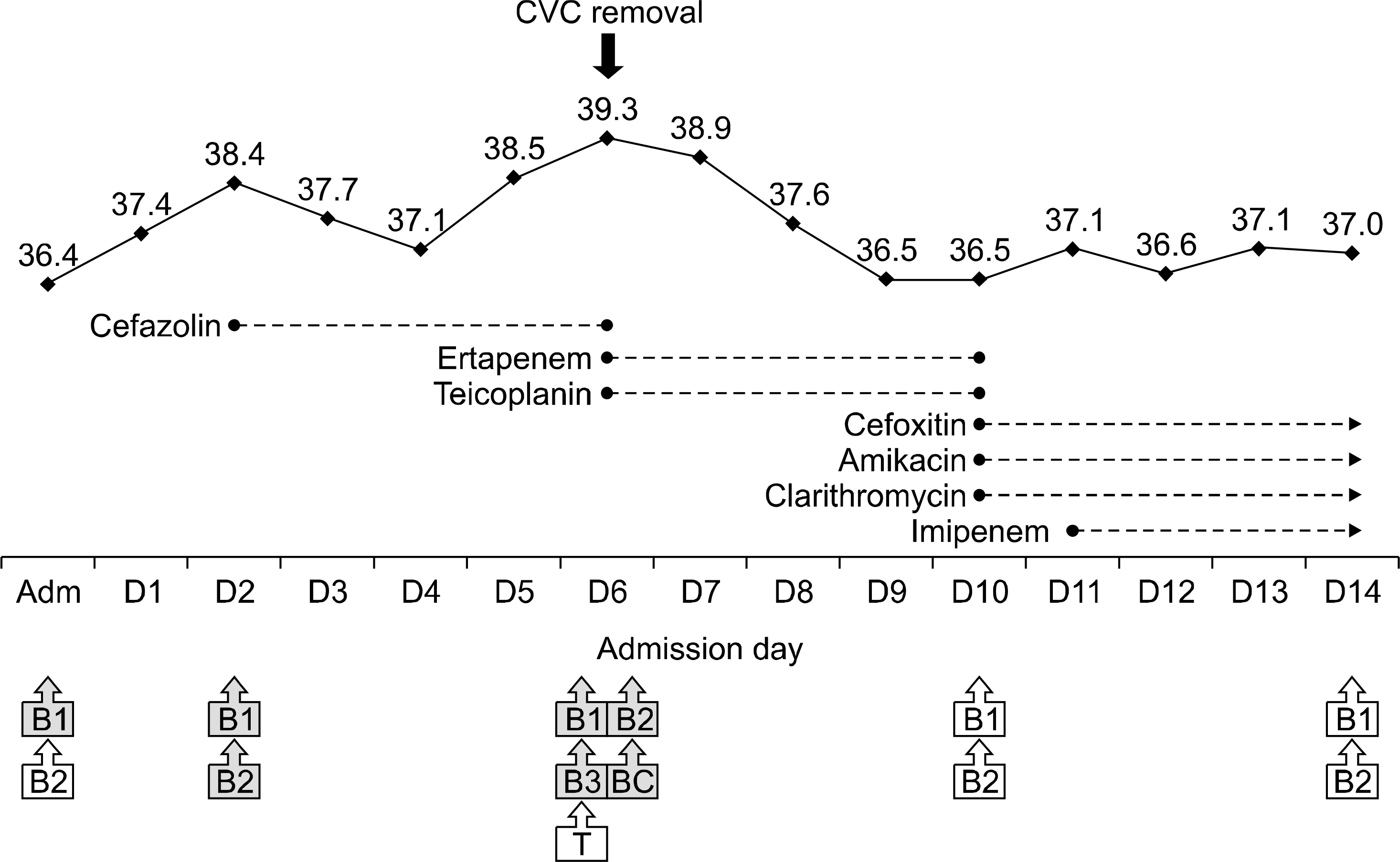
Fig. 1.
Hospital course of patient with Mycobacterium abscessus bacteremia. Solid lines with diamonds indicate body temperature, and dotted lines with circles indicate the period of antimicrobial agent usage. Empty boxes denote negative cultures, and gray-shaded boxes denote positive cultures. Abbreviations: CVC, peripheral central venous catheter; B, peripheral blood culture; BC, catheter blood culture; T, catheter tip culture. Numbers in boxed indicate number of cultures performed on that day.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download